EMPIRICAL GAS LAWS - PowerPoint PPT Presentation
Title:
EMPIRICAL GAS LAWS
Description:
Calculate the density of hydrogen sulfite gas at 587 torr and 56.9 oC. ... 1. Measured at 65 oC and 500.0 torr, the mass of 3.21 L of a gas is 3.5 g. What ... – PowerPoint PPT presentation
Number of Views:172
Avg rating:3.0/5.0
Title: EMPIRICAL GAS LAWS
1
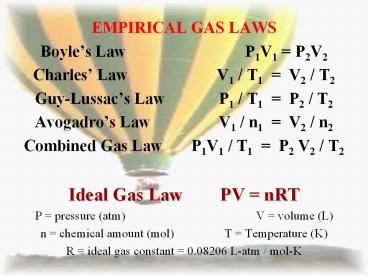
- EMPIRICAL GAS LAWS
- Boyles Law P1V1 P2V2
- Charles Law V1 / T1 V2 / T2
- Guy-Lussacs Law P1 / T1 P2 / T2
- Avogadros Law V1 / n1 V2 / n2
- Combined Gas Law P1V1 / T1 P2 V2 / T2
- Ideal Gas Law PV nRT
- P pressure (atm) V volume (L)
- n chemical amount (mol) T Temperature (K)
- R ideal gas constant 0.08206 L-atm / mol-K
2
Avogadros Hypothesis
- Avogadro pictured the moving molecule as
occupying a small portion of the larger space
apparently occupied by the gas. Thus the
volume of the gas is related to the spacing
between particles and not to the particle size
itself. - Imagine 3 balloons each filled with a different
gas (He, Ar, Xe). These gases are listed in
increasing particle size, with Xe being the
largest atom. According to Avogadros
Hypothesis, the balloon filled with one mole of
He will occupy that same volume as a balloon
filled with one mole of Xe. - So for a gas, the volume and the moles are
directly related. V a n
3
Avogadros Hypothesis
- A sample of N2 gas at 3.0 atm and 20.0oC is known
to occupy a volume of 1.43 L. What volume would
a 0.179 mole sample of NH3 gas occupy at the same
pressure and temperature?
First calculate the number of moles of nitrogen
gas PV nRT where P 3.0 atm, V 1.43
L, R 0.082 L-atm/mol-K, and T 20.0 oC 273
293K n PV / RT (3.0 atm x
1.43L) / (0.082 L-atm/mol-K x 293K)
0.179 moles of N2 So since the moles of N2 is
0.179 mol and the moles of ammonia is 0.179 mol
according to Avogadros hypothesis the volume of
NH3 at that pressure and that temperature is 1.43
L, the same!!!
4
At STP, gas molecules are so far apart that for 1
mole of gas, the overall volume does not
change.STP P 1 atm T 273 K
5
EMPIRICAL GAS LAWS STP 1. The gases in a
rigid Helium filled ball at 25oC exerts a
pressure of 1.2 atm. If the ball is placed in a
freezer and the pressure decreases to 1/8 of its
original value, what is the temperature inside
the ball? 2. A balloon containing 2.50 moles
of He has a volume of 850 mL at a certain
temperature and pressure. How many grams of He
would have to be removed from the balloon in
order for the volume to decrease to 250 mL under
the same conditions? 3. A sample of Ne gas at
21oC 760 mmHg had a volume of 250.0 mL. What
would be the volume of the gas under STP
conditions? 4. How many atoms are present in
1.0 L of SO3 gas at STP?
6
IDEAL GAS LAW
- . What is the pressure inside a gas balloon if it
filled with 852 g of Xe gas at 25.0oC and
occupies a volume of 7.00 L? - P ? 852 g Xe ( 1 mol / 131 g) 6.50 mol
- V 7.00 L T 25oC 273 298 K
- P nRT
- V
- P (6.50 mol) (0.082 L-atm / mol-K) (298 K)
- 7.00 L
- P 22.7 atm
7
IDEAL GAS LAW
- What would be the temperature of 100 g of Ar gas
contained in a 500 L sealed container at 0.8976
atm. - How much would a balloon weigh if it contained
40.0 L of O2 gas at 987 mmHg and 45.3 oC?
T2 1914 oC
mass O2 63.7 g
8
DENSITY OF A GAS
- The density of a gas at STP can be calculated by
- dSTP molar mass/molar volume
- Calculate the density of hydrogen sulfite gas at
STP. - Identify an unknown homonuclear diatomic gas that
was found to have a density of 3.165 g/L at STP.
d (STP) (82 g/mol) / 22.4 L/mol) 3.66 g/L
Cl2
9
DENSITY OF A GAS
- The density of a gas not at STP can be calculated
by - d (MM) P / R T
- Calculate the density of hydrogen sulfite gas at
587 torr and 56.9 oC. - Identify an unknown homonuclear diatomic gas that
was found to have a density of 1.950 g/L at 2.57
atm and 177oC.
d (82 g/mol0.772 atm) / (0.082
L-atm/mol-K330K) 2.34 g/L
N2
10
Properties of Gases
- DIFFUSION
- Diffusion is the ability of two or more gases to
mix spontaneously until a uniform mixture is
formed. - Example A person wearing a lot of perfume walks
into an enclosed room, eventually in time, the
entire room will smell like the perfume.
- EFFUSION
- Effusion is the ability of gas particles to pass
through a small opening or membrane from a
container of higher pressure to a container of
lower pressure. - The General Rule is The lighter the gas, the
faster it moves. - Grahams Law of Effusion
- Rate of effusion of gas A v(molar mass B /
molar mass A) - Rate of effusion of gas B
- The rate of effusion of a gas is inversely
proportional to the square root of the molar mass
of that gas.
11
DALTONS LAW OF PARTIAL PRESSURES
- If there is more than one gas present in a
container, each gas contributes to the total
pressure of the mixture. - Ptotal Pgas A P gas B Pgas C
- If the total pressure of a system was 2.5 atm,
what is the partial pressure of carbon monoxide
if the gas mixture also contained 0.4 atm O2 and
1.48 atm of N2?
PT - PO2 - PN2 PCO 2.5 atm - 0.4 atm - 1.48
atm 0.62 atm
12
STOICHIOMETRY THE GAS LAWS
- 1. Write a balanced chemical equation
- 2. Convert to moles (if gas, use PVnRT or Molar
Volume) - 3. Use the mole ratio to convert from moles of
A to moles of B. - 4. Convert moles of B to desired measurement,
if a gas use PVnRT.
1. What volume of O2 is needed to combust 348.0 L
of C3H8? C3H8 5 O2 ? 3 CO2 4 H2O Due to
Avogadros Hypothesis, the moles of a gas are
directly related to the volume of a gas therefore
it is possible to use the mole ratio on volumes
of gas. 348.0 L C3H8 (5 mol O2 / 1 mol C3H8)
1740 L O2
13
STOICHIOMETRY THE GAS LAWS
2. How many grams of CO2 is produced from 348.0 L
of C3H8 if the temperature is 40.0oC and the
pressure is 654 torr? C3H8 5 O2 ? 3 CO2 4
H2O P 654 torr (1 atm / 760 torr) 0.861
atm T 40oC 273 313 K PV / RT n
(0.861 atm) (348.0 L)
/(0.082 L-atm/mol-K) (313 K)
11.67 mol of C3H8 11.67 mol C3H8 (3 mol CO2
/ 1 mol C3H8) 35.02 mol CO2 35.02 mol CO2
(44 g / 1 mol) 1541 g of CO2
14
STOICHIOMETRY THE GAS LAWS
3. In lab, you decomposed potassium chlorate
into oxygen and potassium chloride. What volume
of O2 at STP can be formed from 3.65 g of
potassium chlorate? 2 KClO3 ? 3 O2 2 KCl 3.65
g (1 mol / 122.6g) 0.02977 mol KClO3 0.02977
mol KClO3 (3 mol O2 / 2 mol KClO3) 0.04466 mol
O2 0.04466 mol O2 ( 22.4 L / 1 mol) 1.00 L
15
PRACTICE PROBLEM 20b
1. Both hydrogen and helium have been used as
buoyant gas in blimps. If a small leak were to
occur, which gas would effuse more rapidly and by
what factor? 2. At STP, 560 mL of a gas has a
mass of 1.08 g. What is the molecular weight of
the gas? 3. A gas is known to be either sulfur
dioxide or sulfur trioxide. Its density at 98oC
and 1.08 atm is 2.84 g/L. Which gas is it? 4.
A 50.0 L cylinder of nitrogen has a pressure of
17.1 atm at 23oC. What is the mass of nitrogen
in the cylinder? 5. When a 2.0 L bottle of
concentrated HCl was spilled, 3.0 kg of CaCO3 was
required to neutralize the spill. CaCO3 (s)
2HCl (aq) ? CaCl2 (aq) H2O (l) CO2
(g) What volume of CO2 gas was released by the
neutralization at 735 mmHg and 20 oC?
Hydrogen effuses first by a factor of 1.41
43.2 g/mol
SO3
986 g
745 L
16
Group Study Problem 20b
1. Measured at 65 oC and 500.0 torr, the mass
of 3.21 L of a gas is 3.5 g. What is the molar
mass of this gas 2. A 100.0 mL sample of air is
analyzed and found to contain 0.835 g N2, 0.0640
g CO2 and 0.197 g O2 at 35 oC. What is the
total pressure of the sample and the partial
pressure of each component? 3. What volume
would 5.30 L of H2 gas at STP occupy if the
temperature was increased to 70oF and the
pressure to 830 torr? 4. Carbon monoxide is
produced by the reaction of coke with oxygen from
preheated air. 2 C O2 ? 2 CO . How many
liters of atmospheric oxygen at an effective
pressure of 182 torr and a temperature of 29.0oC
are required to produce 895 L of carbon monoxide
at 846 torr and 1700oC? 5. Hydrogen gas is
produced by the complete reaction of 8.34 g of
aluminum metal with an excess of gaseous hydrogen
sulfate. How many liters of hydrogen will be
produced if the temperature is 50.0 oC and the
pressure is 0.950 atm?
17
Group Study Problem
Answers 1. 45.9 g/mol 2. PN27.53 atm,
PO21.55 atm, PCO20.380 atm PT 9.46
atm 3. 5.23 L 4. 320 L 5. 12.9 L