Chapter 30: Metamorphic Fluids and Metasomatism - PowerPoint PPT Presentation
Title:
Chapter 30: Metamorphic Fluids and Metasomatism
Description:
Chapter 30: Metamorphic Fluids & Metasomatism Figure 30.30 c. log aCaO - log aSiO2 diagram for the system CaO-MgO-SiO2 -Al2O3 -H2O-CO2 at 425oC, 0.05 GPa, ... – PowerPoint PPT presentation
Number of Views:260
Avg rating:3.0/5.0
Title: Chapter 30: Metamorphic Fluids and Metasomatism
1
Chapter 30 Metamorphic Fluids and Metasomatism
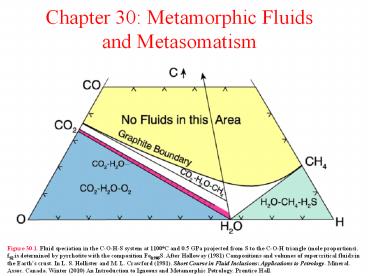
Figure 30.1. Fluid speciation in the C-O-H-S
system at 1100oC and 0.5 GPa projected from S to
the C-O-H triangle (mole proportions). fS2 is
determined by pyrrhotite with the composition
Fe0.905S. After Holloway (1981) Compositions and
volumes of supercritical fluids in the Earth's
crust. In L. S. Hollister and M. L. Crawford
(1981). Short Course in Fluid Inclusions
Applications to Petrology. Mineral. Assoc.
Canada. Winter (2010) An Introduction to Igneous
and Metamorphic Petrology. Prentice Hall.
2
Chapter 30 Metamorphic Fluids and Metasomatism
Figure 30.2. Speciation in C-O-H-S fluids
coexisting with graphite at 0.2 GPa with fO2
buffered by quartz-fayalite-magnetite and fS2
controlled as in Figure 30.1. is the mole
fraction of each species in the fluid. From
Holloway (1981) Compositions and volumes of
supercritical fluids in the Earth's crust. In L.
S. Hollister and M. L. Crawford (1981). Short
Course in Fluid Inclusions Applications to
Petrology. Mineral. Assoc. Canada. Winter (2010)
An Introduction to Igneous and Metamorphic
Petrology. Prentice Hall.
3
Chapter 30 Metamorphic Fluids Metasomatism
Figure 30.3. Variation in the dissociation
constant of NaCl in aqueous solutions with
temperature and pressure. Shaded arrows indicate
regional and contact metamorphic P-T paths. After
Sverjensky (1987) In I. S. E. Carmichael and H.
P. Eugster (eds.), Thermodynamic Modeling of
Geological Materials Minerals, Fluids, Melts.
Rev. in Mineralogy, 17, Mineral. Soc. Amer, pp.
177-209. Winter (2010) An Introduction to Igneous
and Metamorphic Petrology. Prentice Hall.
4
Chapter 30 Metamorphic Fluids Metasomatism
Figure 30.4. Speciation in aqueous-chloride
fluids calculated for an ultramafic bulk
composition assuming a geothermal gradient of
0.1oC/bar. mi is the molality of species i in the
fluid. After Eugster and Baumgartner (1987)
Mineral solubilities and speciation in
supercritical metamorphic fluids. In I. S. E.
Carmichael and H. P. Eugster (eds.),
Thermodynamic modeling of geological materials
Minerals, fluids, melts. Rev. in Mineralogy, 17,
Mineral. Soc. Amer, pp. 367-403. Winter (2010) An
Introduction to Igneous and Metamorphic
Petrology. Prentice Hall.
5
Chapter 30 Metamorphic Fluids Metasomatism
Figure 30.5. Three-dimensional distribution of
fluid about a single grain at q lt 60o (left) and
q gt 60o (right). In the center is a cross section
through a fluid tube at the intersection of three
mineral grains for which q 60o. After Brenan
(1991) Development and maintenance of metamorphic
permeability Implications for fluid transport.
In D. M. Kerrick (ed.), Contact Metamorphism.
Rev. in Mineralogy, 26, Mineral. Soc. Amer, pp.
291-320.
6
Chapter 30 Metamorphic Fluids Metasomatism
Figure 30.5. Selection of time-integrated fluid
fluxes from the literature. Grey area is the
average regional pervasive flow-dominated flux
estimated by Ague (1994a). Sources (a) Breeding
and Ague (2002), (b) Ferry (1992), (c) Ague
(1994b), (d) Oliver et al. (1998), (e)
Chamberlain and Rumble (1989). Range for (e)
computed by Ague (2003) using average flux of 1.5
x 10 m3 m-2 s-1 for 105 and 106 yr. (f) Ague
(1994b) (g) Ague (1997), (h) Dipple and Ferry
(1992), (i) Ferry (1992) and Léger and Ferry
(1993), ( j) Skelton et al. (1995), (k) Walther
and Orville (1982) and Walther (1990). Range for
(k) computed using total timescales of fluid flow
of 106 yr and 107 yr. by Ague (2003), (l) Hanson
(1997), (m) Evans and Bickle (1999). After Ague
(2003).
7
Chapter 30 Metamorphic Fluids Metasomatism
Figure 30.7. A hypothetical column of rock
proposed by J. B. Thompson (1959) Local
equilibrium in metasomatic processes. In P. H.
Abelson (ed.), Researches in Geochemistry. v. 2.
John Wiley. 427-457. pp. 427-457. The left end is
pure periclase and the right end pure quartz.
Between these ends the bulk composition varies
continuously so that the wt. SiO2 increases
linearly from left to right (dashed line). Winter
(2010) An Introduction to Igneous and Metamorphic
Petrology. Prentice Hall.
8
Chapter 30 Metamorphic Fluids Metasomatism
Figure 30.8. A hypothetical column of rock
proposed by J. B. Thompson (1959) Local
equilibrium in metasomatic processes. In P. H.
Abelson (ed.), Researches in Geochemistry. v. 2.
John Wiley. 427-457. pp. 427-457. The left end is
pure periclase and the right end pure quartz.
Between these ends the bulk composition varies
continuously so that the wt. SiO2 increases
linearly from left to right (dashed line). Winter
(2010) An Introduction to Igneous and Metamorphic
Petrology. Prentice Hall.
9
Chapter 30 Metamorphic Fluids Metasomatism
Figure 30.9. Schematic G-XSiO2 diagram for the
SiO2-MgO system at fixed temperature and
pressure. Winter (2010) An Introduction to
Igneous and Metamorphic Petrology. Prentice Hall.
10
Chapter 30 Metamorphic Fluids Metasomatism
Figure 30.10. Expanded view of the monomineralic
enstatite zone in Figure 30.7, showing the
profiles of XSiO2 and mSiO2. Winter (2010) An
Introduction to Igneous and Metamorphic
Petrology. Prentice Hall.
11
Chapter 30 Metamorphic Fluids Metasomatism
Figure 30.11. The hypothetical column of rock of
J. B. Thompson (1959) with the sequences of
mineral assemblages expected to form if diffusion
is effective and the amounts of periclase and
quartz prove inexhaustible. The dashed line shows
the variation in wt. SiO2 across the column and
the lighter dot-dashed lines show the variation
in mSiO2 and mMgO. After J. B. Thompson (1959)
Local equilibrium in metasomatic processes. In P.
H. Abelson (ed.), Researches in Geochemistry. v.
2. John Wiley. 427-457. pp. 427-457. Winter
(2010) An Introduction to Igneous and Metamorphic
Petrology. Prentice Hall.
12
Chapter 30 Metamorphic Fluids Metasomatism
Figure 30.12. aSiO2 aH2O diagram for fluids in
the MgO-SiO2-H2O system at 600oC and 0.2 GPa
calculated using the TQW program (Berman, 1988,
1990, 1991). Winter (2010) An Introduction to
Igneous and Metamorphic Petrology. Prentice Hall.
13
Chapter 30 Metamorphic Fluids Metasomatism
Figure 30.13. SiO2-MgO chemographic diagram
assuming only Qtz, Fo, En, and Per are stable.
Winter (2010) An Introduction to Igneous and
Metamorphic Petrology. Prentice Hall.
14
Chapter 30 Metamorphic Fluids Metasomatism
Figure 30.14. Gresens-type variation diagram
showing the gains or losses (in grams per 100
grams of original rock A) as a function of the
volume factor, fv, in eq. (30.13). Rock A is a
garnet phyllite from Stavanger, Norway, and rock
B is a metasomatized albite schist, supposedly
derived from (A) After Gresens (1967) Chem.
Geol., 2, 47-65. Winter (2010) An Introduction to
Igneous and Metamorphic Petrology. Prentice Hall.
15
Chapter 30 Metamorphic Fluids Metasomatism
Figure 30.15. Isocon diagram of Grant (1986) for
the data from Table 30.1. Some oxides have been
scaled to provide a better distribution of data
points. Econ. Geol., 81, 1976-1982. Winter (2010)
An Introduction to Igneous and Metamorphic
Petrology. Prentice Hall.
16
Chapter 30 Metamorphic Fluids Metasomatism
Figure 30.16. Ideal mineral zonation due to
metasomatism in lt 3-m long ultramafic pods in
low-grade regionally metamorphosed pelites at
Unst, Shetland Islands. After Read (1934)
Mineral. Mag., 23, 519-540. Winter (2010) An
Introduction to Igneous and Metamorphic
Petrology. Prentice Hall.
17
Chapter 30 Metamorphic Fluids Metasomatism
Figure 30.17. Variation in mineral proportions
across the zones between the ultramafic and
quartzo-feldspathic gneiss contact at Grafton,
Vermont, after Sanford (1982). Zone letters at
the top correspond to the zones listed in the
text. Zone letters at the top are A Tlc Ath,
B Tlc, C Act Chl, D transitional, E
quartzo-feldspathic country rock. The vertical
dashed line represents the estimated initial
contact. After Sanford (1982) Amer. J. Sci., 282,
543-616. Winter (2010) An Introduction to Igneous
and Metamorphic Petrology. Prentice Hall.
18
Chapter 30 Metamorphic Fluids Metasomatism
Figure 30.18. AMS diagram (A Al2O3, M MgO
FeO, and S SiO2), projected from K2O, for ideal
lower-temperature metasomatic zones around
ultramafic bodies. After Brady (1977) Geochim.
Cosmochim. Acta, 41, 113-125. Winter (2010) An
Introduction to Igneous and Metamorphic
Petrology. Prentice Hall.
19
Chapter 30 Metamorphic Fluids Metasomatism
Figure 30.19. Hypothetical mM-mSiO2 diagram for
fluids in the AMS system (Figure 30.16). Paths
(1), (2), and (PH) refer to the theoretical
paths in Figure 30.16, and the observed sequence
of Phillips and Hess (1936). After Brady (1977)
Geochim. Cosmochim. Acta, 41, 113-125. Winter
(2010) An Introduction to Igneous and Metamorphic
Petrology. Prentice Hall.
20
Chapter 30 Metamorphic Fluids Metasomatism
Figure 30.20. The same portion of the AMS diagram
as in Figure 30.16, projected from K2O and CaO,
with the locations of analyzed rocks from the
metasomatized zones of Read (1934, see Figure
30.14), reported by Curtis and Brown (1969). The
dashed curve represents a path through the zonal
sequence. After Brady (1977) Geochim. Cosmochim.
Acta, 41, 113-125. Winter (2010) An Introduction
to Igneous and Metamorphic Petrology. Prentice
Hall.
21
Chapter 30 Metamorphic Fluids Metasomatism
Figure 30.21. Schematic representation of major
silicate mineral reactions and component fluxes
associated with metasomatism of the ultramafic
body at Grafton, Vt. Elemental fluxes across
various zones are indicated by the arrows at the
top. Arrows between mineral boxes (somewhat
distorted from the true modes in Figure 30.15)
indicate reactions. When horizontal, these arrows
involve metasomatic reactions when vertical they
are approximately isochemical. The zones listed
at the bottom correspond to those in Figure
30.15, and the heavy dashed line is the estimated
original contact. After Sanford (1982) Amer. J.
Sci., 282, 543-616. Winter (2010) An Introduction
to Igneous and Metamorphic Petrology. Prentice
Hall.
22
Chapter 30 Metamorphic Fluids Metasomatism
Figure 30.22. Variation in chemical potentials of
major components across the metasomatic zones at
Grafton, Vt. Estimated temperature 530oC.
Typical data points and error bars are
illustrated for the MgO profile. Lettered zones
at the top correspond to those in Figure 30.15.
The dashed vertical line is the estimated
original contact. After Sanford (1982). Amer. J.
Sci., 282, 543-616. Winter (2010) An Introduction
to Igneous and Metamorphic Petrology. Prentice
Hall.
23
Chapter 30 Metamorphic Fluids Metasomatism
Figure 30.23. The three principal types of
skarns. From Kerrick (1977) J. Petrol., 18,
144-181. Winter (2010) An Introduction to Igneous
and Metamorphic Petrology. Prentice Hall.
24
Chapter 30 Metamorphic Fluids Metasomatism
Figure 30.23. Chert nodule in carbonate with
layer sequence calcite tilleyite
wollastonite quartz. Christmas Mts., Texas.
From Joesten and Fisher (1988) Geol. Soc. Amer.
Bull., 100, 714-732.
25
Chapter 30 Metamorphic Fluids Metasomatism
Figure 30.25. Schematic isothermal isobaric
mCO2-mH2O diagram for fluids in the CaO-SiO2-H2O
system at high temperatures. After Joesten (1974)
Amer. J. Sci., 274, 876-901. Winter (2010) An
Introduction to Igneous and Metamorphic
Petrology. Prentice Hall.
26
Chapter 30 Metamorphic Fluids Metasomatism
Figure 30.26. Al2O3-CaO-(FeOMgO) diagram
(projected from K2O) showing the mineral phases
and calculated bulk compositional path for
metasomatic zones that develop at the contact
between pelitic and carbonate layers near Lake
Willoughby, VT. Ideal mineral compositions are in
gray, real ones in black. After A. B. Thompson
(1975) J. Petrol., 16, 314-346. Plus signs
represent analyzed bulk-rock compositions within
zones. Winter (2010) An Introduction to Igneous
and Metamorphic Petrology. Prentice Hall.
27
Chapter 30 Metamorphic Fluids Metasomatism
Figure 30.27. Schematic CaO-MgO-SiO2-CO2-H2O
diagram showing the composition of the fluid
solution in equilibrium with the phases shown at
approximately 600oC and 0.2 GPa (projected from
H2O and CO2 at a constant 11 ratio). After
Frantz and Mao (1976) Amer. J. Sci., 276,
817-840. Winter (2010) An Introduction to Igneous
and Metamorphic Petrology. Prentice Hall.
28
Chapter 30 Metamorphic Fluids Metasomatism
a
1 cm
b
1 cm
Figure 30.28. a. Metasomatic zones separating
quartz diorite (bottom) from marble (top).
Zonation corresponds to third row from bottom in
Table 30.1. b. Symmetric metasomatic vein in
dolomite. Zonation corresponds to last row in
Table 30.1. Adamello Alps. After Frisch and
Helgeson (1984) Amer. J. Sci., 284, 121-185.
Photos courtesy of Hal Helgeson. Winter (2010) An
Introduction to Igneous and Metamorphic
Petrology. Prentice Hall.
29
Chapter 30 Metamorphic Fluids Metasomatism
Figure 30.29. Mineral zones and modes developed
at the contact between quartz diorite and
dolomitic marble in Figure 30.26a. Initial
contact may be at either side of the contact
zone. Index numbers at the top indicate the
locations of bulk chemical analyses. After Frisch
and Helgeson (1984) Amer. J. Sci., 284, 121-185.
Winter (2010) An Introduction to Igneous and
Metamorphic Petrology. Prentice Hall.
30
Chapter 30 Metamorphic Fluids Metasomatism
Figure 30.30. a. log aCaO - log aSiO2 diagram in
the system CaO-MgO-SiO2-H2O-CO2 at 425oC, 0.05
GPa, and XCO2 0.007. Numbered points correspond
to the index numbers in Figure 30.27. After
Frisch and Helgeson (1984) Amer. J. Sci., 284,
121-185. Winter (2010) An Introduction to Igneous
and Metamorphic Petrology. Prentice Hall.
31
Chapter 30 Metamorphic Fluids Metasomatism
Figure 30.30. b. log aCaO - log aMgO diagram in
the system CaO-MgO-SiO2-H2O-CO2 at 425oC, 0.05
GPa, and XCO2 0.007. Numbered points correspond
to the index numbers in Figure 30.27. After
Frisch and Helgeson (1984) Amer. J. Sci., 284,
121-185. Winter (2010) An Introduction to Igneous
and Metamorphic Petrology. Prentice Hall.
32
Chapter 30 Metamorphic Fluids Metasomatism
Figure 30.30 c. log aCaO - log aSiO2 diagram for
the system CaO-MgO-SiO2 -Al2O3 -H2O-CO2 at 425oC,
0.05 GPa, and XCO2 0.007. Numbered points
correspond to the index numbers in Figure 30.27.
After Frisch and Helgeson (1984) Amer. J. Sci.,
284, 121-185. Winter (2010) An Introduction to
Igneous and Metamorphic Petrology. Prentice Hall.
33
Chapter 30 Metamorphic Fluids Metasomatism
Figure 30.31. Zonation in an experimental skarn
formed at the contact between granodiorite and
limestone at 600oC, Pfluid 0.1 GPa (XCO2
0.07). After Zharikov, V.A. and G.P. Zaraisky
(1991) Experimental modeling of wall-rock
metasomatism. In L. L Perchuck (ed.), Progress in
Metamorphic and Magmatic Petrology. A Memorial
Volume in Honor of D. S. Korzhinskii. Cambridge
University Press. Cambridge, pp. 197-245. Photo
courtesy G. Zaraisky. Winter (2010) An
Introduction to Igneous and Metamorphic
Petrology. Prentice Hall.
34
Chapter 30 Metamorphic Fluids Metasomatism