Titration of a weak base with a strong acid - PowerPoint PPT Presentation
1 / 8
Title:
Titration of a weak base with a strong acid
Description:
Slide 1. Lecture 17. Titration of a weak base with a strong acid ... fOH H2O fO- H3O 2H2O H3O OH- Kw = [H3O ][OH-] Making the usual assumptions, ... – PowerPoint PPT presentation
Number of Views:119
Avg rating:3.0/5.0
Title: Titration of a weak base with a strong acid
1
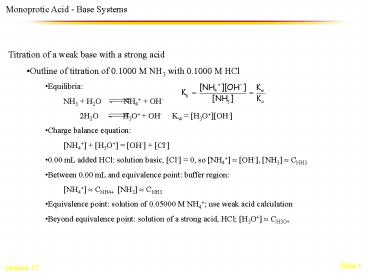
Monoprotic Acid - Base Systems
- Titration of a weak base with a strong acid
- Outline of titration of 0.1000 M NH3 with 0.1000
M HCl - Equilibria
- NH3 H2O NH4 OH-
- 2H2O H3O OH- Kw
H3OOH- - Charge balance equation
- NH4 H3O OH- Cl-
- 0.00 mL added HCl solution basic, Cl- 0, so
NH4 ? OH-, NH3 ? CNH3 - Between 0.00 mL and equivalence point buffer
region - NH4 ? CNH4 NH3 ? CNH3
- Equivalence point solution of 0.05000 M NH4
use weak acid calculation - Beyond equivalence point solution of a strong
acid, HCl H3O ? CH3O
2
Monoprotic Acid - Base Systems
- pH of dilute solutions of very weak acids
- Example 5 x 10-5 M phenol, fOH
- fOH H2O fO- H3O
- 2H2O H3O OH- Kw
H3OOH- - Making the usual assumptions,
- fO- ? H3O, fOH ? CfOH,
- A more complete analysis
- Charge balance fO- OH- H3O- ?
- Mass balance CfOH fO- fOH but fO- ltlt
fOH - Using Ka
3
Monoprotic Acid - Base Systems
- Acid - base indicators are weak acids or bases
whose conjugate acid has a different color from
the conjugate base. - color 1 color 2
- HIn1 H2O In-1 H3O
- In-2 H2O HIn2 OH-
- Color 2 predominates if In-1 10 x HIn1 or
HIn2 10 x In-2 - Color 1 predominates if HIn1 10 x In-1 or
In-2 10 x HIn2 - For indicator system 1,
- color 1
- color 2
- Likewise for indicator system 2, color changes
over pH range pKb ?1
?olor changes from color 1 to color 2 over a
pH range pKa 1
4
Monoprotic Acid - Base Systems
- Acid - base indicators
- See Table 10-1, FAC7 p. 191, for transition range
of various acid base indicators, the pKa of the
indicators and the color change of each
indicator - See inside front cover of FAC7 for a colorful
chart of indicators - Types of indicators
- Phthalein indicators
- phenolphthalein
5
Monoprotic Acid - Base Systems
- Acid - base indicators
- Types of indicators
- Sulfonphthalein indicators bromocresol green
6
Monoprotic Acid - Base Systems
- Acid - base indicators
- Types of indicators
- Azo indicators methyl orange (methyl red has
-COO- in place of -SO3-)
7
Monoprotic Acid - Base Systems
- The shape of titration curves and choosing an
indicator - Strong acid - strong base see Fig. 10-4. FAC7 p.
98 for indicator choices including the effect of
reagent concentrations - Weak acid - strong base see Figs. 10-11 and
10-12, FAC7 p. 213 for indicator choices
including the effects of reagent concentrations
and Ka - Weak base - strong acid see Fig. 10-13, FAC7 p
216 for indicator choices including the effects
of Kb - Dissecting the titration curve of weak acids with
strong base or weak base with strong acid - Initial pH weak acid or weak base calculation
- Between initial pH and equivalence point pH of a
solution containing a weak acid and its
conjugate base or weak base and its conjugate
acid - Buffer calculation use Henderson-Hasselbach
equation or a modification if the solution is
dilute - Equivalence point calculation pH of a solution
of the conjugate base of the weak acid or
conjugate acid of the weak base - Beyond the equivalence point pH of a solution
containing a strong base or strong acid
8
Monoprotic Acid - Base Systems
- Buffers are solutions that resist change in pH as
a result of dilution of the solution or the
addition of an acid or base to the solution - Consider a solution prepared by mixing 50.00 mL
Hac with 10.00 mL NaOH
?ompare this to diltuting a pH 4.16 solution of
HCl by 10,000 H CHCl 6.92 x 10-6 H ?
CHCl H 1.00 x 10-7 pH7.00