Chapter 13 NMR Spectroscopy - PowerPoint PPT Presentation
1 / 50
Title:
Chapter 13 NMR Spectroscopy
Description:
Radio waves supply the energy. to flip from low energy to. high energy state. ... along with Hb and Hd. Chapter 13. 40. F. 13C NMR - and now for something ... – PowerPoint PPT presentation
Number of Views:71
Avg rating:3.0/5.0
Title: Chapter 13 NMR Spectroscopy
1
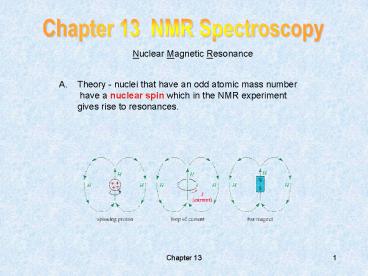
Chapter 13 NMR Spectroscopy
Nuclear Magnetic Resonance
- Theory - nuclei that have an odd atomic mass
number - have a nuclear spin which in the NMR experiment
- gives rise to resonances.
2
- In the presence of a strong (very strong)
magnetic - field there are then two arrangements
- The resonances are sharp for nuclear spin 1/2,
otherwise they are - frequently broad for spin gt 1/2 nuclei
(quadrapolar effects).
3
2. The energy difference between these two spin
states is very tiny - 1.9 x 10-5 kcal/mol! For a
typical NMR spectrometer ( magnetic field
strength 4.7T)
4
3. In actual fact the situation is not so simple
- there Are really populations of spin-up and
spin- down nuclei
The differences between spin-up and spin-down
nuclei are on the order of parts per million.
5
B. The NMR experiment 1. Experimental design
Radio waves supply the energy to flip from low
energy to high energy state.
2. As implied by the drawing - not all 1H
nuclei absorb at the same rf field strength
6
Position of nucleus Circulation of electrons
7
There are actually two factors which make the 1H
nuclei absorb at different field strengths a.
Electron density at the nucleus (the proton).
b. Circulation of p electron clouds
This is a big factor
8
3. All chemical shifts (the different field
strengths for absorbtion of radiation) are
relative to TMS - tetramethylsilane
chemically inert liquid lots of 1Hs -
small conc. needed very shielded protons
9
4. Information from 1H NMR experiment
- The number of absorbtions tells us how many
different - kinds of protons there are in the molecule, e.g.
10
b. The chemical shift implies what kind of
group(s) are connected to the carbon or other
atom that the proton is bonded to, e.g.
11
c. The area under the absorbtions tells us the
relative number of protons that correspond to
that absorbtion, e.g.
4.59
1.53
12
12
d. The splitting of the absorbtions tells us how
many protons are three bonds away from the
proton(s) which correspond to the absorbtion.
13
5. Whole body NMR - MRI (Magnetic Resonance
Imaging) Primarily relaxation rates of 1Hs in
H20.
14
MRI
MRI
standard x-ray
15
tumors!!
16
(No Transcript)
17
(No Transcript)
18
C. A return to 1H NMR chemical shifts - general
situations 1.alkanes and derivatives
shielded
deshielded
19
b. Alkenes, aromatics and alkynes - ring currents
20
c. Some examples
You will always get something like Table 13-3
in your book.
21
D. Nuclear Spin Coupling 1. Theory
nonequivalent (chemically different) protons
connected on the same carbon atom or connected
on adjacent carbons are said to be magnetically
coupled, i.e.
The magnetic coupling in 1,1,2-tribomoethane then
creates
22
Jab
JabJba
Jba
Jba
23
So we get
Lets take a related example CH3CH2-I
24
For the methyl protons - they see
For the methylene protons - they see
So the resultant spectrum is
25
2. General cases of 1H-1H nuclear spin coupling
lines N 1 where n number of adjacent
protons
26
The magnitude of the coupling constants are
generally
27
3. Complications associated with nuclear spin
coupling. a. the field strength of the
spectrometer
28
b. Chemical shift differences between Ha and Hb
29
(No Transcript)
30
c. Different spins with different coupling
constants
31
An example
(doublet of doublets)
CH3 group
Ethyl group
32
d. Rapid bond rotations or motions that make
protons equivalent
33
e. Rapid proton exchange for N-H and O-H protons
- why these kind of protons have
chemical shifts that are variable and show no
coupling
wet EtOH
pure EtOH
34
E. Nonequivalent protons/groups of protons - some
concerns(!)
Stereotopicity compares identical atoms or groups
within a molecule
1. An atom (center) is prochiral if the exchange
of one of its equivalent substitutents for
something else would create a chiral center.
Ha and Hb in the molecule above are said to be
enantiotopic. They can be differentiated by
chiral means chiral solvents or reagents because
their interactions with chiral agents are
diasteromeric
35
A prochiral center in a molecule which also
possesses a chiral center leads to a different
result. Consider R malic acid
Normally they can be differentiated by any
physical method.
The two atoms are diastereotopic they are
different all of the time
36
Lets take a look at the conformations of R malic
acid
This is probably the most stable confomer
Hydrogen bonding possible
37
The 1H NMR of R malic acid
38
Suppose we had a racemic mixture of R and S
malic acid - how many signals would we see for
the methylene protons? (a) In an achiral
solvent
(b) In a chiral solvent we would see all 4
signals.
39
If substitution of equivalent groups leads to
identical molecules, the groups are homotopic.
Ha and Hb are enantiotopic along with Hc and Hd
Ha and Hc are homotopic along with Hb
and Hd
40
F. 13C NMR - and now for something completely
different 1. General properties a. Many oher
nuclei have a spin of 1/2 31P, 19F, 15N,
195Pt, etc but the most important from our
point of view is 13C b. 12C - no nuclear
spin - 98.89 natural abundance c. 13C - spin
1/2 - 1.11 abundance actually about 100
times less sensitive than 1H NMR 2. Chemical
shifts - similar pattern to 1H NMR BUT about 10
times the range!, i.e.
41
(No Transcript)
42
3. Nuclear spin coupling a. No 13C-13C coupling
- 1.11 times 1.11 about 1 chance in 10,000
that there will be two adjacent 13C atoms b.
There is 13C-1H coupling - this causes troubles
(S/N) so normally the 1H nuclei are decoupled -
each 13C absorbtion is a single resonance
1H decoupled
1H coupled
(solvent)
(solvent)
13C NMR counts the number (and kinds) of
carbon atoms
43
c. Rather than getting a 1H coupled NMR we can do
a trick - off resonance decoupled NMR This
then tells us how many protons are connected to
each carbon atom (splitting N1) d. NOTE -
peak intensity or area under the peak is
NOT proportional to the relative number of carbon
atoms. This is stuff that we will not cover!
You will always get tables of IR frequencies,
1H and 13C chemical shifts
44
G. Work through the problems in the book! Lets
take a couple of examples
1.
Prob. CO
45
Three kinds of carbons
CO !!
46
-CH2CH3 group!!
-CH3 group !!
Three kinds of carbons
CO !!
47
C9H8O2
2.
deg. of unsat. Z 1/2(2C 2 -H) (count O
atoms as zero) Z 1/2(182-8) 1/2(12) 6!!
Aromatic (?) Z4
-OH ?
Z1
Note we account for 2 O and 9 carbons if there
is a phenyl group and one CC
48
C9H8O2
-OH ?
4 carbons
2 carbons
ALL CONSISTENT!!
49
So I have 1. -OH
3.
4.
2.
C9H8O2
with two Hs connected
olefinic Hs
There are just three possibilities!!
50
olefinic Hs
This is not so good since the two vinyl Hs are
not too different
-or-