MSE 550 CVD - PowerPoint PPT Presentation
1 / 67
Title: MSE 550 CVD
1
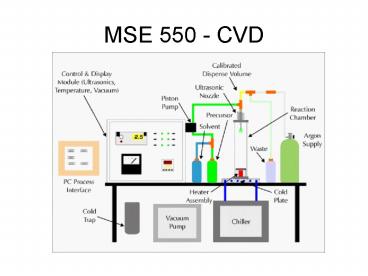
MSE 550 - CVD
2
Chemical Vapor Deposition
3
- Chemical vapor deposition (CVD) is a widely used
method for depositing thin films of a large
variety of materials. Applications of CVD range
from the fabrication of microelectronic devices
to the deposition of protective coatings. In a
typical CVD process, reactant gases (often
diluted in a carrier gas) at room temperature
enter the reaction chamber. The gas mixture is
heated as it approaches the deposition surface,
heated radiatively or placed upon a heated
substrate. Depending on the process and operating
conditions, the reactant gases may undergo
homogeneous chemical reactions in the vapor phase
before striking the surface. Near the surface
thermal, momentum, and chemical concentration
boundary layers form as the gas stream heats,
slows down due to viscous drag, and the chemical
composition changes. Heterogeneous reactions of
the source gases or reactive intermediate species
(formed from homogeneous pyrolysis) occur at the
deposition surface forming the deposited
material. Gaseous reaction by-products are then
transported out of the reaction chamber.
4
What is CVD?
- Thin film formation from vapor phase reactants.
Deposited films range from metals to
semiconductors to insulators. - An essential process step in the manufacturing of
microelectronic devices. High temperatures and
low pressures are the most common process
conditions, but are not necessary. - All CVD involves using an energy source to break
reactant gases into reactive species for
deposition.
5
Chemical Vapor Deposition CVD is the formation
of a film on a surface from a volatile precursor
(vapor or gas), as a consequence of one or more
chemical reactions which change the state of the
precursor. Many different films can be deposited
elements and compounds, crystalline,
polycrystalline, and amorphous. Most films can be
deposited from several different precursor
systems. Plasma discharges can be used to help
things along, or the substrate and/or the gas can
be heated or cooled. Different deposition
techniques, process conditions, and treatment
after deposition produce films with
differing characteristics, suitable for different
applications. Each film has an optimal set of
characterization techniques. In every case, CVD
processes must provide a volatile precursor
containing the constituents of the film
transport that precursor to the deposition
surface encourage or avoid reactions in the gas
phase encourage surface reactions that form the
film and do it rapidly, reproducibly, and
uniformly for industrial applications.
6
- Advantages
- high growth rates possible
- can deposit materials which are hard to evaporate
- good reproducibility
- can grow epitaxial films
- Disadvantages
- high temperatures
- complex processes
- toxic and corrosive gasses
7
(No Transcript)
8
(No Transcript)
9
(No Transcript)
10
(No Transcript)
11
Examples of CVD
- Metals/Conductors - W, Al, Cu, doped poly-Si
- Insulators (dielectrics) - BPSG, Si3N4, SiO2
- Semiconductors - Si, Ge, InP, GaAsP
- Silicides - TiSi2, WSi2
- Barriers TiN, TaN
12
(No Transcript)
13
(No Transcript)
14
(No Transcript)
15
(No Transcript)
16
(No Transcript)
17
- Types of CVD reactions
- Pyrolysis - thermal decomposition
- AB(g) ---gt A(s) B(g)
- ex Si deposition from Silane at 650 C
- SiH4(g) ---gt Si(s) 2H2(g)
- use to deposit Al, Ti, Pb, Mo, Fe, Ni, B, Zr, C,
Si, Ge, SiO2, Al2O3, MnO2, BN, Si3N4, GaN,
Si1-xGex, . . . - Reduction
- often using H2
- AX(g) H2(g) ltgt A(s) HX(g)
- often lower temperature than pyrolysis
- reversible gt can use for cleaning too
- ex W deposition at 300 C
- WF6(g) 3H2(g) ltgt W(s) 6HF(g)
- use to deposit Al, Ti, Sn, Ta, Nb, Cr, Mo, Fe,
B, Si, Ge, TaB, TiB2, SiO2, BP, Nb3Ge, Si1-xGex,
. . . - Oxidation
- often using O2
- AX(g) O2(g) ---gt AO(s) OX(g)
- ex SiO2 deposition from silane and oxygen at 450
C (lower temp than thermal oxidation) - SiH4(g) O2(g) ---gt SiO2(s) 2H2(g)
18
(No Transcript)
19
(No Transcript)
20
(No Transcript)
21
- Compound formation
- often using ammonia or water vapor
- AX(g) NH3(g) ---gt AN(s) HX(g)
- AX(g) H2O(g) ---gt AO(s) HX(g)
- ex deposit wear resistant film (BN) at 1100 C
- BF3(g) NH3(g) ---gt BN(s) 3HF(g)
- use to deposit TiN, TaN, AlN, SiC, Al2O3, In2O3,
SnO2, SiO2, . . . - Disproportionation
- compounds involving elements with multiple
valence states - 2AB(g) ltgt A(s) AB2(g)
22
- use to deposit Al, C, Ge, Si, III-V compounds, .
. . - Reversible Transfer
- ex use to deposit GaInAs, AlGaAs, InP, FeSi2, .
. .
23
- Mass transport in gas
- goals
- deliver gas uniformly to substrate (uniform
films) - optimize flow for maximum deposition rate
- Two flow regimes
- Molecular flow
- diffusion in gas
- D T3/2 / P from Kinetic Theory of Gasses
- reduce Pressure for higher D and higher
deposition rate - Viscous flow
- low flow rates produces laminar flow (desired)
- high flow rates produces turbulent flow (avoid)
laminar flow simple case flow past a plate
near plate velocity 0 gt stagnant layer
24
- diffuse gas through stagnant layer to surface
- mass transport depends on
25
(No Transcript)
26
Generic CVD Steps
- Five steps occur during all CVD processes
- Reactants pumped through reactor.
- Reactants diffuse across boundary layer to
surface. - Reactants adsorb on surface (adatoms).
- Surface reactions pos. formation of islands or
clusters. - Pos. surface diffusion of adatoms.
- Diffusion of by-products away from surface.
27
(No Transcript)
28
- Simple model (Grove, 1967)
- AB(g) ---gt A(s) B(g)
- F1 flux to surface
- F2 flux consumed in film
- CG concentration of AB in gas
- CS concentration of AB at surface
- F1 hG (CG - Cs)
- where hG gas diffusion rate constant
- F2 kS Cs
- where kG surface rate constant
- in steady state F1 F2 F
29
- NOTE Two rate-limiting cases
- mass transfer limited
- small hG
- growth controlled by transfer to substrate
- hG is not very temperature dependent
- common limit at higher temperatures
- surface reaction limited
- small kS
- growth controlled by processes on surface
- adsorption
- decomposition
- surface migration
- chemical reaction
- desorption of products
- kS is highly temperature dependent (increases
with T) - common limit at lower temperatures
- often preferred
30
(No Transcript)
31
variations along flow direction
Consider flow into and out of a volume (as in
Chapter 1)
apply boundary conditions
32
- all gas reacts at substrate
- C 0 at y 0
- initial concentration is constant
- C Ci at x 0
- no flow out of the top
- dC/dy 0 at y b
- SOLVE differential equation subject to these
boundary conditions - C(x, y) a mess (see equation 4-41)
- ASSUME large flow rate or large chamber
- vaveb gtgt D¹
- examine this solution
- proportional to Ci
- at y b, sin 1
- C decreases exponentially with x
- tricks to improve uniformity
- tilt substrate into flow
- increase T continuously along x
33
Variables Affecting Steps
- Diffusion of reactants to by products away from
surface. - Gas flow rates, pressure, reactor configuration
- boundary layer formation
- Temperature
- diffusion is a mildly activated process
- Surface reaction and surface diffusion
- Temperature (both are affected exponentially by
temperature) - Surface interactions (reactive and overall
sticking coefficient of a species) - Rate of incoming reactants and out going
by-products
34
Molecule/Surface Interactions
- Physisorption
- No electron transfer occurs between adatom
surface - Binding energies 0.1 eV
- Essentially condensation
- Substrate/adsorbate independent
- Doesnt occur at temperatures much above the
boiling point of the adsorbate - Chemisorption
- Electronic interactions between surface
adspecies - Binding energies 1 eV
- This is a chemical reaction
- Substrate/adsorbate selective
35
Nucleation and Growth
- Initial formation of clusters (nuclei)
- Can grow or shrink until a critical size is
reached - Above critical size, increasing nuclei size
lowers the overall surface energy - Clusters can grow at the expense of
sub-critically sized clusters - Clusters impinge and grow together to form
continuous film (coalescence)
36
(No Transcript)
37
(No Transcript)
38
Limiting Cases
- Assuming steps occur sequentially, then the
slowest step determines the deposition rate. - Reactions
- Mass Transport
39
(No Transcript)
40
(No Transcript)
41
(No Transcript)
42
Surface Reactions
- Reaction rate strong function of temperature
- Reactant species must first be adsorbed on
substrate (wafer) for deposition to take place. - After adsorption, the reactive species can remain
fixed at the surface site or migrate along the
surface. This is temperature dependent and
affects step coverage.
43
Mass Transport Limited Depositions
- Processes are run in laminar flow regime
- Re?UL/? lt 2100
- Boundary layer forms next to wafer surfaces
- Due to viscous forces in the gas
- Reactants must diffuse across the boundary layer
to reach wafer surface - Boundary layer thickness decreases with
increasing gas velocity - Rate increases with decreasing boundary layer
- Maximizes when reaction becomes reaction rate
limited
44
(No Transcript)
45
Why Epi?
- Bipolar devices
- Add a lightly doped layer on a heavily doped
substrate increases breakdown voltage of the
collector/substrate junction - Deposited over patterned heavily doped areas
called buried layers - CMOS
- No SiOx precipitates, unlike substrates
- Less junction leakage due to defects
- Smoother surface leads to better gate oxide
46
(No Transcript)
47
(No Transcript)
48
(No Transcript)
49
(No Transcript)
50
(No Transcript)
51
Silicon Precursors
- SiCl4- Silicon tetrachloride (1150-1250 C)
- SiHCl3-Tricholorosilane (1110-1150 C)
- SiH2Cl2-Dichlorosilane (1020-1120 C)
- SiH4- Silane (650-900 C)
- Gas phase nucleation a problem
- Si2H6- Disilane (400-600 C)
- Pyrogenic and toxic
52
Doping an Epi Layer
- Add dopant gas to deposition reaction
- PH3, AsH3, B2H6
- Added in ppm levels in H2
- Outdiffusion from doped substrate
- High deposition temperature drives diffusion out
of highly doped substrates into growing epi films
- Auto doping
- Deposition temperature evaporates dopants from
substrates or from chamber walls - Dopants are then incorporated in growing film
53
(No Transcript)
54
- Types of pressure dependent CVD processes
- Atmospheric Pressure CVD (APCVD)
- Sub-atmospheric CVD (SACVD)
- Low Pressure CVD (LPCVD)
- In general, APCVD is controlled by the rate of
reactant transport to the wafer, LPCVD is
controlled by the reaction rate
55
(No Transcript)
56
(No Transcript)
57
(No Transcript)
58
LPCVD
- Lower Pressure
- Increases diffusivity by about 1000x
- Increases boundary layer thickness by (?P) 0.5
(factor of about 25 for 1 torr vs. 760 torr) - Results in a net mass transport increase by more
than an order of magnitude - Reaction rate limited
- Temperature control critical
59
Depletion Effects
- Batch reactors prone to reactant depletion
- Due to deposition on wafers nearer the gas
entrance - Increase gas flow
- Reduces fraction of reactant depleted
- Distributed feed
- Adds fresh reactant along length of reactor
- Temperature gradient
- Increase temperature along length to compensate
for lower reactant concentration - Can significantly affect film properties (grain
size, stress, etc.
60
PECVD
- Film growth involves surface reactions with
incoming gas radical molecules. - Most radicals are electrically neutral so their
transport to the wafer surface is by gas phase
diffusion. - Radical concentration gradient drives the
diffusion process.
61
PECVD Process Control Parameters
- RF power, frequency, and bias
- Gas composition and flow rate
- System pressure and reactant partial pressure
- Deposition time
- Temperature
62
(No Transcript)
63
(No Transcript)
64
(No Transcript)
65
(No Transcript)
66
(No Transcript)
67
(No Transcript)