Ionic Compounds and Metals - PowerPoint PPT Presentation
Title:
Ionic Compounds and Metals
Description:
Ionic Compounds and Metals Types of bonds A chemical bond is the force that holds atoms together in a molecule. Ionic bonds form when electrons are transferred ... – PowerPoint PPT presentation
Number of Views:193
Avg rating:3.0/5.0
Title: Ionic Compounds and Metals
1
Ionic Compounds and Metals
2
Types of bonds
- A chemical bond is the force that holds atoms
together in a molecule. - Ionic bonds form when electrons are transferred
between atoms. - Covalent bonds form when electrons are shared
between atoms.
3
Electronegativity
- Electronegativity is a measure of the attraction
a bonded atom has for electrons. - Electronegativity increases from bottom to top
and from left to right.
4
Formation of Ionic Bonds
- If the electronegativity difference is large (a
metal with a nonmetal) an ionic bond will form. - Ionic bonds are the strongest type of bond.
- Substances resulting from ionic bonds are
classified as crystalline solids.
5
Lets Practice
- Identify the type of bond (ionic or covalent)
that will form between each pair of atoms. - Na and Cl
- C and O
- N and H
- Ca and O
- K and N
- Mg and F
6
Example
7
Forming Ions
- When neutral atoms gain or lose valence e- an ion
is formed. - Ions are atoms with a charge
- Cations have a positive charge (K, Li)
- Anions have a negative charge (F-, I-)
8
Ions forming bonds
- Cations are attracted to anions because of
opposite charges. - Opposites attract
9
Why do ions form?
- Ions form to attain a more stable electron
configuration. - Remember that a Noble gas configuration is the
most stable - Ne - ___ ___ ___ ___ ___
- 1s 2s 2p
- All Noble Gases have a full octet (8 valence e-)
10
Noble Gases
11
Why do ions form?
- Na __ __ __ __ __ __
- 1s 2s 2p 3s
- Na __ __ __ __ __ __
- 1s 2s 2p 3s
- Now Na looks like neon
- Ne __ __ __ __ __
- 1s 2s 2p
- All atoms are trying to have a Noble Gas electron
configuration so, they will lose or gain
electrons to get there
12
Why do ions form?
- F __ __ __ __ __
- 1s 2s 2p
- F- __ __ __ __ __
- 1s 2s 2p
- Ne __ __ __ __ __
- 1s 2s 2p
13
Review
- Draw the dot notation for each element and the
charge of its ion - N
- S
- Ba
- Li
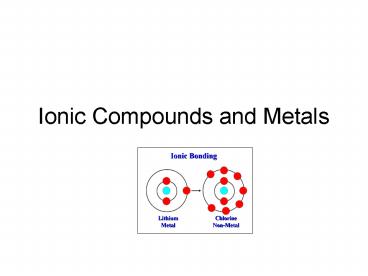
14
Ionic Bonds
- Ionic bonds- the force that holds two oppositely
( and -) charged particles together. - Ionic compounds contain ionic bonds and are
neutral - Usually forms between a metal and a nonmetal
15
Forming Ionic Bonds
16
Forming Ionic Compounds
- Write an equation showing how ionic compounds are
formed for the following elements. - Na and I
- Mg and O
- Al and Cl
17
Properties of Ionic CompoundsShape
- The Ionic bond produces unique characteristics
for ionic compounds - Crystals are created by repeating patterns of
cations and anions. This balances the forces
between the ions. - This is called a crystal lattice
18
Properties of Ionic CompoundsPhysical Properties
- Electrolytes (aqueous ions- Ions dissolved in
water) conduct electricity - Solid ionic compounds DO NOT conduct electricity
because the charges are locked in place
19
Properties of Ionic CompoundsPhysical Properties
- Ionic solids are strong and hard to break apart
- High Melting points
- High Boiling points
- Transition metals in the crystal give the ionic
solid color.
20
Properties of Ionic CompoundsPhysical Properties
- The solid ionic crystals are hard, rigid, and
brittle - The strong forces between ions produce this
property
21
Ionic Compounds
- Ionic compounds are repeating patterns of the
cation and anion. - The simplest ratio of ions involved is called a
formula unit. - NaCl is the formula unit for table salt
22
Ions in Ionic Compounds
- Monatomic ions- ions made of only one type of
atom (Cl-, F-, Li) - Polyatomic ions- ions made of at least two
different types of ions (OH-, NH4) - Cation Anion ? Ionic Compound
23
Writing formulas for ionic compounds
- Binary ionic compounds contain a single cation
and anion. Remember that the final compound must
be neutral so the charges must balance. - Ca2 Cl- ? CaCl2
24
Cris-Cross method
- Record the ion symbol and charge
- The of the charge of the cation becomes the
subscript for the anion and the of the charge
for the anion becomes the subscript for the
cation. The 1 does not have to be recorded it
is understood. - Al3 O-2 ? Al2O3
25
Cris-Cross method
- Al2O3 is the formula units for aluminum oxide.
The subscripts indicate how many ions are needed
of each in order to balance the charges.
26
Try a few
- Potassium and Iodide
- Magnesium and chloride
- Aluminum and bromide
- Cesium and nitride
27
Naming Ionic Compounds
- NaBr
- Name the cation then the anion. Cations are
always written first - Monatomic cations always keep their name. If the
cation is an ion that may carry multiple charges
then a roman numeral is used to indicate which
charge the cation is carrying. Fe3 (III) and
Fe2 (II).
28
Naming Ionic Compounds
- The monatomic anions ending is changed to -ide.
Fluorine is changed to Fluoride. - NaBr- sodium bromide
- Fe3N2 Iron (II) nitride
29
Lets practice
- CaCl2
- K2O
- CuCl2
- Fe2O3
30
What about Polyatomic ions?
- Al3 and PO4-3 ? AlPO4
- The same rules apply, however NEVER CHANGE A
SUBSCRIPT THAT IS ALREADY THERE. - Polyatomic ions may also require () around them
if there is a need for more than one to balance
the charge