Kinetics of Elementary Reactions - PowerPoint PPT Presentation
1 / 13
Title:
Kinetics of Elementary Reactions
Description:
Kinetics of Elementary Reactions A reaction is elementary if it takes place in a single irreducible act at the molecular level, just the way it is written in the ... – PowerPoint PPT presentation
Number of Views:151
Avg rating:3.0/5.0
Title: Kinetics of Elementary Reactions
1
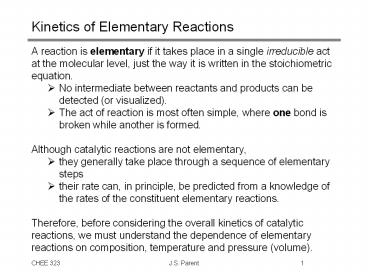
Kinetics of Elementary Reactions
- A reaction is elementary if it takes place in a
single irreducible act at the molecular level,
just the way it is written in the stoichiometric
equation. - No intermediate between reactants and products
can be detected (or visualized). - The act of reaction is most often simple, where
one bond is broken while another is formed. - Although catalytic reactions are not elementary,
- they generally take place through a sequence of
elementary steps - their rate can, in principle, be predicted from a
knowledge of the rates of the constituent
elementary reactions. - Therefore, before considering the overall
kinetics of catalytic reactions, we must
understand the dependence of elementary reactions
on composition, temperature and pressure (volume).
2
Elementary Reactions
- Since an elementary reaction represents a
molecular event, its equation may not be written
arbitrarily, but the way it takes place. - With this restriction, the molecularity of the
reaction is identical to its stoichiometry.
3
Theories of Elementary Reaction Kinetics
- The rates of even the simplest reactions are very
difficult to calculate from first principles. In
engineering practice, you will rely on
experimental data. - While the basic science of reaction kinetics is
not sufficiently developed for design purposes,
existing models of reaction dynamics provide a
means of understanding reaction phenomena,
analyzing experimental data, and extrapolating
knowledge to other systems. - Atkins details three approaches to the
calculation of rate constants - Collision Theory
- Transition State Theory
- Molecular Reaction Dynamics
- We will examine the collision and transition
state theories.
4
Transition State Theory - Elementary Reactions
- Transition state theory is founded on the
expectation that during the transition from
initial reagents to final products, an activated
complex of higher energy is formed. - This transition state is not an intermediate,
but a unique configuration of the system in
transit from one state to another. - Although this activated complex is inherently
unstable, we often assume that it possesses
thermodynamic properties (albeit ill-defined),
and propose molecular structures.
5
Transition State of an SN2 Reaction
- You have likely seen the concept of a transition
state in CHEM 288, where nucleophilic
substitution reactions were introduced. - In the example below, the alkoxide ion is the
nucleophile (Lewis base) displaces iodide, the
weaker base. - The reaction is believed to be bimolecular,
passing through a transition state as drawn
below - Clearly this transition state is not a stable
compound, and therefore is not a reaction
intermediate, but an activated complex.
6
Potential Energy Surface for Hydrogen Exchange
- Owing to the complexity of potential energy
calculations, one of the only systems to be
analyzed is that of collinear hydrogen exchange.
7
Potential Energy of the Reaction Coordinate
8
Transition State Theory - Thermodynamic
Formulation
- The Rate of an Elementary Step
- The number of elementary acts per unit time is
determined the number of systems passing through
the activated complex configuration. - We express the elementary reaction as
- At equilibrium, the activated complex Xy will be
in equilibrium with the reactants and products,
and the concentration can be calculated from
thermodynamic principles. - Where q is the reference concentration, usually 1
mole/litre. - Transition state theory assumes that even when
the system is not at equilibrium, activated
complexes are at equilibrium with the reactants.
9
Transition State Theory - Thermodynamic
Formulation
- Based on this assumption, the concentration of
the activated complex is derived from a
thermodynamic treatment - q unit concn
- which, can be expressed in terms of the relative
Gibbs energy of the activated complex, - DGy represents the free energy of activation.
- The difference between the Gibbs energy of the
activated complex, and the Gibbs energies of the
reactants at the reference state - This represents the free energy barrier to
reaction that includes both potential energy (DH)
and conformational restrictions (DS).
10
Transition State Theory - Thermodynamic
Formulation
- The rate of the forward elementary reaction
- is expressed as
- q unit concn
- where n is the frequency of vibration of the
activated complex in the mode that corresponds to
decomposition into products. - This is the frequency of the molecular vibration
which leads the complex to dissociate into
products C and D. - For this diatomic reaction, statistical mechanics
assigns - sec-1
- where kb Boltzmanns constant 1.3806610-23
J/K - T reaction temperature, K
- h Plancks constant 6.626210-34 J s
11
Transition State Theory - Thermodynamic
Formulation
- With a measure of the decomposition frequency,
the rate of our elementary reaction takes the
form - Given our elementary rate expression for the
reaction, - The rate constant, k, for the reaction is
identifiable as - q unit concn
- which ends our development of transition state
theory. It correctly predicts the orders of the
reaction, provides a means of interpreting the
observed rate in terms of enthalpic and entropic
contributions, and provides guidelines into the
temperature dependence of k.
12
Temperature Dependence of Elementary Reactions
- The variation of elementary reaction rate
constants with temperature is almost always
expressed as - The term Ea is usually called the activation
energy, although interpretations of this quantity
differ between specific theories of reaction
rate. The temperature exponent, m, does
likewise. - m 0 corresponds to classical Arrhenius theory
- m 1/2 is predicted by collision theory
- m 1 is generated by transition state theory
- In practice, the dependence of the
pre-exponential factor on temperature is usually
much weaker than that of the activation energy. - If gathered under kinetic control, reaction rate
data plotted as ln(k) versus 1/T or ln(k/T)
versus 1/T is usually linear.
13
Temperature Dependence of Elementary Reactions