BBC - PowerPoint PPT Presentation
1 / 3
Title:
BBC
Description:
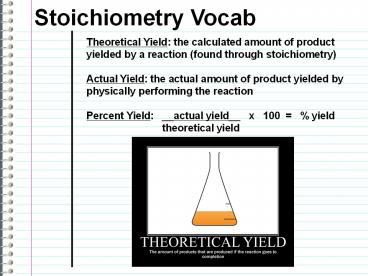
Stoichiometry Vocab Theoretical Yield: the calculated amount of product yielded by a reaction (found through stoichiometry) Actual Yield: the actual amount of product ... – PowerPoint PPT presentation
Number of Views:101
Avg rating:3.0/5.0
Title: BBC
1
Stoichiometry Vocab
Theoretical Yield the calculated amount of
product yielded by a reaction (found through
stoichiometry) Actual Yield the actual amount
of product yielded by physically performing the
reaction Percent Yield __actual yield__ x
100 yield
theoretical yield
2
Stoichiometry Vocab
Molar Mass the mass obtained by adding up the
weights of all the atoms in a compound Given
the value (a number) that is given in a
stoichiometry problem look for a number and then
moles or grams ex 3.0 moles of CH4 or 23.0
g of NaCl Wanted the quantity that the problem
is asking you to find look for How many or
Calculate ex How many grams of CH4 are
produced Calculate the moles of NaCl
required
3
Stoichiometry Vocab
Limiting Reactant (Limiting Reagent) the
reactant that limits how much product you can
make can change depending on how much of each
reactant you have Excess/Sufficient
usually refers to reactants not a factor in
stoichiometry since it means you have more than
enough and it will not be a limiting
reactant Mole Ratio (Molar Ratio) ratio of any
substances in a balanced equation used to
convert one quantity in a reaction to another