Homonuclear PowerPoint PPT Presentations
All Time
Recommended
Title: PowerPoint Presentation Author: Mary Beth Smith Last modified by: student Created Date: 10/1/2006 8:02:39 PM Document presentation format: On-screen Show (4:3)
| PowerPoint PPT presentation | free to view
14.5 The structure of diatomic molecules (f) The structures of homonuclear ... Composition of orbitals in homonuclear molecules (6 MB MBQuick-Time with music) ...
| PowerPoint PPT presentation | free to view
Shape of molecular orbitals in homonuclear diatomic molecules ... The Occupation of homonuclear diatomic orbitals(PDF) (From the Wilson Group, ...
| PowerPoint PPT presentation | free to view
MO diagram for homonuclear diatomic molecules Li2 through N2. MO diagram for homonuclear diatomic molecules O2 and F2. N2. Lewis diagram :N ...
| PowerPoint PPT presentation | free to download
In this case wo = 0, because. we are on-resonance. ... Since the peaks are skewed exactly 45 degrees, we can ... 90 pulse by 180 degrees (90-y), and the ...
| PowerPoint PPT presentation | free to view
Diatomic: molecule contains two atoms. large difference in. electronegativity. Covalent bonding ... Homonuclear Diatomic Molecules ...
| PowerPoint PPT presentation | free to view
Chapter 9 Covalent Bonding: orbitals Topics Hybridization and the localized electron model The molecular orbital model Bonding in homonuclear diatomic molecules ...
| PowerPoint PPT presentation | free to download
158 a Lecture 23. H2 Valence Bond Theory & Extensions. Simple MO of Homonuclear Diatomics ... Apply to homonuclear diatomics. Fred J. Grieman. H2 Results. E ...
| PowerPoint PPT presentation | free to view
Zumdahl s Chapter 9 Covalent Bonding Orbitals Chapter Contents Hybridization and LE dnspm Molecular Orbitals Bond Order Bonding in Homonuclear Diatomics ...
| PowerPoint PPT presentation | free to download
Molecular Orbital (MO) Theory. Diagram of molecular energy ... Bonding in s-block homonuclear diatomic molecules. Energy. Li2. Be2. Combinations for p-orbitals ...
| PowerPoint PPT presentation | free to view
... with homonuclear. couplings, homonuclear correlation ... Homonuclear correlation (continued) Since the I to S or S to I polarization transfers are the ...
| PowerPoint PPT presentation | free to view
Diatomic molecules: The bonding in H2 ... Diatomic molecules: Homonuclear Molecules of the Second Period ... Diatomic molecules: MO diagrams for Li2 to F2 ...
| PowerPoint PPT presentation | free to download
Allows 'indirect' probing of vibrational states in molecules. Unique spectral signatures provide means of ... Homonuclear diatomic molecules are Raman active ...
| PowerPoint PPT presentation | free to view
Before mixing. After mixing. t1. t2. The Power of 2D NMR: ... Differ Only By The Nature Of Mixing. Scalar Coupling. Dipolar Coupling. Homonuclear. COSY ...
| PowerPoint PPT presentation | free to view
Properties of the diatomic ideal gas. eatomic ... Rotational energy of a diatomic ... Only if the diatomic is heteronuclear such as CO, s =2 for a homonuclear ...
| PowerPoint PPT presentation | free to view
The 1s orbital is much smaller than the 2s orbital ... Bonding in Homonuclear Diatomic Molecules ... Heteronuclear Diatomic Species ...
| PowerPoint PPT presentation | free to view
considered, so for the diatomic molecules from Li2 to F2, the overlaps ... Energy levels of first-row homonuclear diatomic molecules (H&S Fig 1.23) ...
| PowerPoint PPT presentation | free to view
Bond polarity in diatomic and polyatomic molecules. Molecular Orbitals in Solids ... Bond lengths in homonuclear diatomic molecules are used to define the covalent ...
| PowerPoint PPT presentation | free to view
The Bohr one electron atom as a starting point for the electron configurations ... Orbital correlation diagrams. Homonuclear and heteronuclear diatomic molecules ...
| PowerPoint PPT presentation | free to download
For diatomic molecules in Pd 2, Lewis models show all electrons are paired, ... MO DIAGRAM: homonuclear diatomic. Molecules = Li2 to N2. 2p. 2p. 1s. 1s. 1s. 1s ...
| PowerPoint PPT presentation | free to download
Ch 5 Lecture 3 Group Theory and MO's. The Group ... Homonuclear diatomic molecules (nonpolar) H2 Ne2. Heteronuclear diatomic molecules (polar) C O, LiF-ionic ...
| PowerPoint PPT presentation | free to view
Microwave Spectroscopy or Rotational Spectroscopy Applied Chemistry Course: CHY101 Sun Lamp X-ray Table lamp, Tube light (400 nm -800 nm or 400 790 THz) Microwave ...
| PowerPoint PPT presentation | free to download
What do you expect the multiplicity to be for Acetone-d6 (C3D6O) ... COSY Correlation Spectroscopy. TOCSY Total Correlation Spectroscopy ...
| PowerPoint PPT presentation | free to view
Nuclear Magnetic Resonance (NMR) Spectroscopy Dr. Vincent J. Storhaug Deuterated Solvent Signals in 13C NMR Spectra Why do you see this as a triplet at 77 ppm?
| PowerPoint PPT presentation | free to view
... spectrum of fluctuations. For a randomly fluctuating field: Exponential ... Lorentzian - Power available from fluctuations at a given frequency. Substitute for ...
| PowerPoint PPT presentation | free to download
8-2.5 Molecular Orbital Theory Molecular orbital theory describes covalent bonds in terms of molecular orbitals, which result from interaction
| PowerPoint PPT presentation | free to view
Honors Chemistry Chapter 10: Chemical Bonding II 10.1 Molecular Geometry Study of the shapes of molecules Molecule s geometry affects properties Valence shell ...
| PowerPoint PPT presentation | free to download
sigma & pi molecular orbitals from 2s and 2p. bonding and antibonding s & p molecular ... Chalkboard. MO's from p orbitals. First consider symmetry. z axis ...
| PowerPoint PPT presentation | free to download
Molecular Orbital Theory. Combination of atomic orbitals on different atoms forms molecular orbitals (MO's) ... Waves that describe atomic orbitals have both ...
| PowerPoint PPT presentation | free to view
HCN BIMA Image. C4H BIMA Image. Why do molecules form? Classical Argument ... Electronic orbital angular momenta precess about internuclear axis due to strong ...
| PowerPoint PPT presentation | free to download
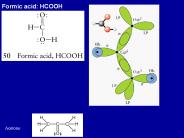
Formic acid: HCOOH. Acetone. Benzene C6H6. Resonance structures; each point corresponds to a CH ... Each C is sp2 hybridized, one of the sp2 forming a s-bond ...
| PowerPoint PPT presentation | free to download
Title: PowerPoint Presentation Author: Serdyuk Last modified by: Igor Created Date: 1/15/2005 6:02:40 AM Document presentation format: Company
| PowerPoint PPT presentation | free to download
Acquire. p /2. General Two Dimensional Experiment. Fourier Transform. t1 - f1 and t2 - f2 ... Acquire. p /2. t1. t2. Vary t1. Collect a series of 1D spectra ...
| PowerPoint PPT presentation | free to view
Title: 1D 1H NMR spectra of proteins Author: Matt Cordes Last modified by: Matt Cordes Created Date: 2/5/2002 12:08:08 PM Document presentation format
| PowerPoint PPT presentation | free to view
Which response lists only the molecules given below that are not paramagnetic? ... lists all the following diatomic molecules and ions that are paramagnetic? ...
| PowerPoint PPT presentation | free to view
An atom or molecule is diamagnetic if it contains only ... Molecular orbital theory vs VSEPR-valance bond theory. Two differing explanations of bonding. ...
| PowerPoint PPT presentation | free to download
Electronic Structure of Molecules AST380E Yancy L. Shirley E sg1s Excited States of H2 L = 0 S = 0 X1Sg+ su1s sg2s su2s pu2p sg2p L = 0 S = 1 b3Su+ L = 0 S = 0 B1Su ...
| PowerPoint PPT presentation | free to download
NAMING ELEMENTS The chemical symbol and the name of the elements must be known before starting any discussion in chemistry. There is a correlation between the name ...
| PowerPoint PPT presentation | free to download
Harmonic Oscillator Harmonic Oscillator IR spectroscopy is an important tool in structural determination of unknown compound CO2, A greenhouse gas ?
| PowerPoint PPT presentation | free to download
OPOLE Opole University Institute of Physics, Plasma Spectroscopy Group * I am from.. Symmetry of Gas inlet-outlet Experiment parameters Spectra registered in 6545 ...
| PowerPoint PPT presentation | free to download
Crystallography vs. NMR advantage/disadvantages. Experimental difficulties ... need good crystals for crystallography. need 13C and 15N label for NMR ...
| PowerPoint PPT presentation | free to download
trigonal bipyramidal. octahedral. Cl. Cl. Be. BF3. CH4. PCl5 ... trigonal pyramidal. 9. Class # of atoms. bonded to central atom # lone. pairs on central atom ...
| PowerPoint PPT presentation | free to download
REAPDOR sequence for measuring dipolar couplings between I 1 ... Investigating reaction products. Chemical shift information. ppm. 13C-CP-MAS. Tolane sample ...
| PowerPoint PPT presentation | free to view
Nuclei observable by NMR. Why some nuclei have no spin ? ... AxXx. AyXy. Multispin systems - product operators. Spectrum of a AX spin system. Spectrum of A ...
| PowerPoint PPT presentation | free to view
Thousands of interactions between atoms- also need to be assigned ... More information to identify signals. Lower sensitivity to MW of protein ...
| PowerPoint PPT presentation | free to download
Molecular Orbital Theory or when electrons don t like sitting between atoms!
| PowerPoint PPT presentation | free to download
Title: PowerPoint Presentation Author: Matt Cordes Last modified by: Matt Cordes Created Date: 2/6/2005 7:57:34 PM Document presentation format: On-screen Show
| PowerPoint PPT presentation | free to view
Title: PowerPoint Presentation Last modified by: admin Created Date: 1/1/1601 12:00:00 AM Document presentation format: (4:3) Other titles
| PowerPoint PPT presentation | free to download
I. Diatomic Molecules. 1. ... I. Diatomic Molecules. 2. Symmetry and designation of electronic states of ... I. Diatomic Molecules. 3. Born-Oppenheimer ...
| PowerPoint PPT presentation | free to view
LACC Chem101 * Kinetic Molecular Theory of Gases an explanation of the properties of an ideal gas in terms of the behavior ... Calculate the rms speed of CO and SF6 ...
| PowerPoint PPT presentation | free to download
... NOESY, ROESY 5.4.3 1D Selective Excitation 5.4.4 1D Selective Excitation 6.1 13C NMR 12C has no magnetic spin. 13C has a magnetic spin, ...
| PowerPoint PPT presentation | free to download
Title: Slide 1 Author: GWH28-DGCMP-P6RC4-6J4MT-3HFDY Last modified by: GWH28-DGCMP-P6RC4-6J4MT-3HFDY Created Date: 3/12/2006 11:30:36 PM Document presentation format
| PowerPoint PPT presentation | free to download
... so in sample some spins precess faster than the Larmor frequency, ... J coupling causes MAXa and MAXb to precess at different rates. In the rotating frame: ...
| PowerPoint PPT presentation | free to download
Use of Genetic Algorithm for Quantum Information Processing by NMR V.S. Manu and Anil Kumar Centre for quantum Information and Quantum Computing
| PowerPoint PPT presentation | free to view
Title: PowerPoint Presentation Author: Prof.C.R.Raj Last modified by: P.Pramanik Created Date: 6/12/2006 11:58:26 AM Document presentation format
| PowerPoint PPT presentation | free to view