The surface science approach - PowerPoint PPT Presentation
1 / 42
Title:
The surface science approach
Description:
We want to have setailed information on important. parameters like: ... Find KA from isostere: HOW? IC. T. IC-5 /42 Lecture-8 18-11-2004. 2. Order Adsorption ... – PowerPoint PPT presentation
Number of Views:132
Avg rating:3.0/5.0
Title: The surface science approach
1
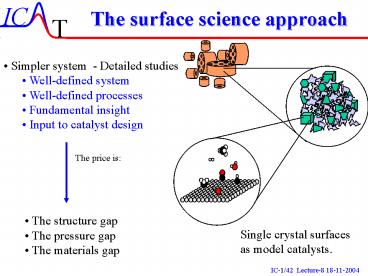
The surface science approach
- Simpler system - Detailed studies
- Well-defined system
- Well-defined processes
- Fundamental insight
- Input to catalyst design
The price is
- The structure gap
- The pressure gap
- The materials gap
Single crystal surfaces as model catalysts.
2
Determination of important parameters.
We want to have setailed information on
important parameters like
- Adsorption rates meaning S(T)S0e-Ea/RT
- Reaction rates kk0e-Er/RT
- Desorption rates
kdeskdes0e-Edes/RT
Construct a microkinetic model based on
reliable fundamental data resulting in deep
insigth.
3
1. Order Adsorption
By equalizing the chemical potentials of the gas
and the surface and introducing the appropriate
partition function we found
We must now estimate S0(T) from experiments or
calculations
4
1. Order Adsorption
We remember that for desorption
Find KA from isostere HOW?
Langmuir isotherm for adsorption on a single site
5
2. Order Adsorption
6
2. Order Adsorption
However if BA so it is A2
7
Determination of sticking coefficients
- Thermalized Experiments
- The real data, but not always possible and
they do not reveal details - Beam experiments
- Are giving detalied information on the
dependence of energy in - different degrees of freedom, but may not
probe the correct - reaction pathway
8
1. order adsorption
9
1. order adsorption
S01/D0
D0
10
2. Order adsorption
11
Uptake on Clean Ru(0001)
Agreement with S0(10.8)10-12 at 300K in
earlier work Dietrich, Geng, Jacobi, and Ertl, J.
Chem. Phys. 104 (1) (1996) 375
12
Minority sites may rule the game
13
Some typical S0(T)
14
Determination of desorption rates
- The bonding energy of simple adsorbates to a
specific surface - On-set temperatures of reaction or decomposition
- Reaction pathways on the surface
15
1. Order desorption
16
The Complete Method
As the intercept and the slope. This can be done
for all qleft Giving n and Edes as function of
coverage.
17
2. Order desorption
18
The simple method 2. order
Notice symmetric and shifts down
Thus by assuming q1/2 can we again estimate Edes
qleft1/2
19
N2 desorption from Ru(0001)
0.05 ML Au on Ru(0001)
Clean Ru(0001)
Mass 14 QMS signal
Mass 14 QMS signal
- All thermal experiments with N2/Ru(0001) systems
are dominated - by steps.
20
More complex behaviour
A typical behaviour for a two state situation
But a dublet can also be due to strong lateral
interaction i.e. Erepulsion Eo for qgt1/2
21
TPD for identification of reaction pathways
Tells a lot about surface reactions
Can be used for analysis
22
Micro-kinetic Modeling
The idea is to collect all the fundamental
information I.e. any adsorption rates, desorption
rates incl. prefactors and activation energies,
sticking coefficients etc. and put them into a
detailed model.
If correct the model should then be capable of
describing the process and identify what is
important in the process, i.e. what is the rate
limiting step, how can it be changed and what is
the coverage of various species.
23
Micro-kinetic Modeling Ammonia Synthesis
24
The Ammonia Synthesis
We can now express each coverage in terms of K,
k, pressure, and q
25
The Ammonia Synthesis
26
The Ammonia Synthesis
27
The details of ammonia synthesis
Notice how the molecular state is not in play
since it is assume in equilibrium qN2 cancels
28
The rate limiting step may not even have an
barrier
The important message is that although the
activation is low there is still an entropy
barrier
29
Comparison Theory Experiment
30
Example Nitrogen coverage
31
Now back to the model
K promoted ammonia catalyst at 673 K, 100 bar
approaching 68 of the equilibrium ammonia
concentration.
32
Approach towards Equilibrium
The exit concentration is 19 and corresponds to
75 of the obtainable equilibrium conversion for
100 bar and 673K.
The approach towards equilibrium is slow because
N blocks the surface
33
Comparrision of model and exp.
This is not a proof but an indication that we are
on the right track
34
Structural gap in the Ammonia Synthesis over Ru
Catalysts
Why Ruthenium?
Fe is blocked by N (NH3)
Fe
NH3 Conc.
Ru is not as easily blocked by N (NH3), but by
Hydrogen and it is expensive!!
Fe
Reactor length
35
Energy Diagram for N2 Dissociation
Terrace site
Step site
Electronic effects account for one third of the
barrier change Geometrical effects for
two thirds of the barrier change
S. Dahl, A. Logadottir, R. Egebjerg, J. Larsen,
I. Chorkendorff, E. Tørnqvist, and J. K. Nørskov,
Phys. Rev. Lett. 83 (1999) 1814.
Energy
36
Microkinetic Model for Ammonia Synthesis over
Ruthenium
From assuming 1 of active sites on the clean
Ru(0001) surface. k1 105exp(-37 kJ/mol / RT)
(bar-1s-1)
The rest of the parameters are in agreement with
tailing edge of TPD spectra of N2, H2 and NH3
desorption from Ru(0001)
S. Dahl, J. Sehested, J. H. Jacobsen, E.
Tornqvist, and I. Chorkendorff, J. Catal. 192
(2000) 391.
37
Universality
Fe(111) is good
Ru steps are good
You can now see why the catalyst bed should be
varied down through the reactor
38
The Industrial Ammonia synthesis
The Real Ammonia reactor
The schematic reactor
39
The Industrial Ammonia synthesis
40
(No Transcript)
41
Promoters
Structural promotors Al2O3 and CaO for the
ammonia cat. helps stabilizing the structure
ensuring a huge surface area
Electronic promotors K, Cs for the ammonia
cat. Set up a dipole moment lowing the activation
energy for either adsorption or desorption
They may also act as inhibitors i.e. methane
sticking on Ni
42
Inhibitors or poisons
Poisons may as just seen work through an
electronic effect
Often poisons are species that just block sites,
i.e. bond Strongly or irreversible to the active
sites.
Good examples are Sulfur, Chlorine, and
Oxygen 1 ppm H2O in the ammonia syn-gas reduces
the activity by a factor of 2.
Explain why one should not use leaded fuel on a
modern car?