Applications of Neutralization Titrations - PowerPoint PPT Presentation
1 / 3
Title:
Applications of Neutralization Titrations
Description:
0.1 M can be boiled under reflux for 1 hour without acid loss ... Constant boiling HCl can be weighed and diluted to a known volume to prepare ... – PowerPoint PPT presentation
Number of Views:157
Avg rating:3.0/5.0
Title: Applications of Neutralization Titrations
1
- Applications of Neutralization Titrations
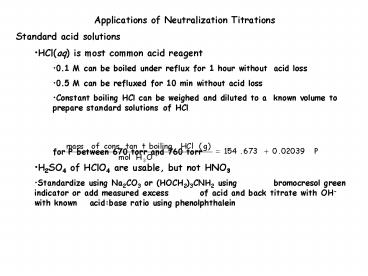
- Standard acid solutions
- HCl(aq) is most common acid reagent
- 0.1 M can be boiled under reflux for 1 hour
without acid loss - 0.5 M can be refluxed for 10 min without acid
loss - Constant boiling HCl can be weighed and diluted
to a known volume to prepare standard solutions
of HCl - for P between 670 torr and 760 torr
- H2SO4 of HClO4 are usable, but not HNO3
- Standardize using Na2CO3 or (HOCH2)3CNH2 using
bromocresol green indicator or add measured
excess of acid and back titrate with OH- with
known acidbase ratio using phenolphthalein
2
- Applications of Neutralization Titrations
- Standard base solutions
- NaOH most common
- Reasonably stable if protected from air and glass
- Store in polyethylene or parafin coated glass
- Effect of CO2
- CO2 2OH- CO32- H2O
- 1 mol CO2 ? 2 mol OH- ? 2 mol H
- if an indicator with acid color transition is
used - CO32 2H H2CO3 no error in base
consumed - if an indicator with a base color transition
is used - CO32- H HCO3- introduce a
determinate error
3
- Applications of Neutralization Titrations
- Preparation of NaOH standard solutions
- Purchase 50 NaOH in which Na2CO3 is insoluble
- Pre-prepared solutions are available - Acculute
- Boil the distilled water to remove CO2
- Standardization
- Use potassium biphthalate - molar mass 204
- KH(IO3)2 - a strong acid with molar mass 390
- Kjeldahl determination of N
- Carbonate mixtures