Chapter 15 Molecular Luminescence Spectrometry - PowerPoint PPT Presentation
1 / 40
Title:
Chapter 15 Molecular Luminescence Spectrometry
Description:
Phosphorescence. Figure 15-3 ... phosphorescence so no fluorescence is seen. 2) sample measured at liquid N2 temperature (-196 oC) ... – PowerPoint PPT presentation
Number of Views:652
Avg rating:3.0/5.0
Title: Chapter 15 Molecular Luminescence Spectrometry
1
Chapter 15 Molecular Luminescence Spectrometry
Page 357
2
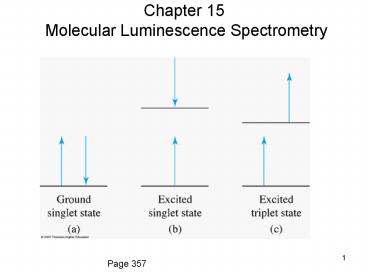
Figure 15-1
3
Processes that compete with fluorescence
- 1) vibrational relaxation
- 2) internal conversion
- 3) predissociation
- 4) dissociation
- 5) external conversion
- 6) intersystem crossing
- 7) phosphorescence
4
Figure 15-1
5
Vibrational relaxation
- much faster than fluorescence
- fluorescence occurs at wavelengths longer than
absorbed radiation, except were no vibrational
relaxation can take place - Seen as l'r and lr
- resonance fluorescence - labsorbed lemitted
6
Internal conversion
- processes that allow molecule to drop to lower
electronic E state without emission of photons - occurs by overlap of lowest vibrational level in
a higher vibrational level with an upper
vibrational level in a lower E electronic state
which then leads to vibrational relaxation - can occur between
- 2 or more upper electronic states (S1 and S2 in
Fig. 15-1) - lowest excited electronic state and electronic
ground state (S1 and S0 in Fig. 15-1). - rate constant kic
7
Predissociation
- result of internal conversion when electron moves
to upper vibrational level in a lower electronic
state that can cause bond rupture - rate constant kpd
8
Dissociation
- initial absorption promotes electron to upper
vibrational level in excited electronic state
that causes immediate dissociation - rate constant kd
9
External Conversion
- E transfer between excited molecule and solvent
or other solutes - rate constant kec
10
Intersystem Crossing
- spin of electron is reversed (singlet to triplet)
- most common causes
- molecules containing I or Br (heavy atom effect)
or in solutions containing these atoms (or ions) - O2 or other paramagnetic species
- rate constant ki
11
heavy atom effect on fluorescence of quinine
12
Phosphorescence
- Can only occur after intersystem crossing
- is much slower than fluorescence
13
Quantum yield
- quantum yield f quanta emitted
quanta absorbed - f kf
kf ki kec kic kpd kd
14
Transitions that cause fluorescence
- n to p and p to p
- p to p has best quantum efficiency because
- it has shortest excited state lifetime -
10-7-10-9s vs. 10-5-10-7 for n to p (larger kf) - intersystem crossing less likely
- s to s often causes predissociation and
dissociation
15
Other factors that affect fluorescence
- 1) structure
- 2) temperature
- 3) viscosity
- 4) pH
16
Structure
- fused ring aromatic is best
- conjugated systems next best
- rigid structure best Thought to reduce internal
conversion
17
Temperature and Viscosity
- lower T leads to higher fluorescence
- higher viscosity leads to higher fluorescence
- both reduce rate of external conversion
18
pH
- acidic or basic substituents can cause change in
fluorescence as pH changes - protonation or deprotonation of acidic or basic
functional groups can cause changes in number of
resonance structures
19
Effect of pH change on quinine fluorescence
20
Effects of concentration on fluorescence
- Fluorescence is directly proportional to
concentration at low concentrations but shows
negative deviation at higher concentrations for 3
reasons - 1) selfquenching excited molecules collide and
deactivate in similar fashion to external
conversion. Collisions of excited analyte
molecules are more likely at higher
concentrations - 2) self-absorption (inner filter effect) if
excitation and emission spectra overlap, then
some of emitted radiation can be reabsorbed by
ground state analyte molecules.
21
Self-absorption
Figure 15-5
22
Effects of concentration on fluorescence
- 3) theoretical as seen below, higher order
terms in equation relating concentration and
fluorescence cause negative deviations - PF kfF(P0 P) (1)
- PF power of fluorescence signal
- (P0 P) amount of light absorbed by analyte
- k is an instrumental term composed of two terms
- k f(?)g(l).
- f(?) is a measure of how much of emitted light
the instrument collects, and g(l) is detector
response at emission l
23
Effects of concentration on fluorescence
- A ebC
- - log (P/P0) ebC
- P/P0 10?ebC fraction of
light transmitted - 1 (P/P0) 1 ? 10?ebC fraction of light
absorbed - P0 - P P0(1 ? 10?ebC) actual amount
absorbed (2)
24
Effects of concentration on fluorescence
- substituting equation 2 into equation 1
- PF kfFP0(1 ? 10 ?ebC)
- kfFP02.303ebC ? (2.303ebC)2
(2.303ebC)3?etc - 1! 2!
3! - if 2.303ebC lt 0.05, higher order terms drop out
and equation reduces to - PF 2.303ebCkfFP0
- at higher concentrations, higher order terms
cannot be neglected and they cause negative
deviations
25
differences between PF kfFP0(1 ? 10 ?ebC) and A
ebC
- 1) PF ? P0, so signal can be increased by
increasing P0. - Not possible for absorption measurements
because - A log(P0/P), and as P0 is increased, P
increases by same relative amount, so ratio is
unchanged. - 2) k term can be increased by more efficient
collection of light. - Cannot be done for absorbance measurement.
- 3) Because of 1 and 2 there is no fixed maximum
fluorescence to which instrument can be set. (A
instrument is set to T 0). - A standard (quinine) must be used to compare
results from different instruments. - 4) Fluorescence signal is easily amplified.
Absorbance cannot be increased by amplification.
26
Fluorometer and Spectrofluorometer components
- Sources
- 1) low P Hg lamp useful in fluorometers. Emits
many lines in UV and VIS. - 2) Xe lamp continuous and more powerful used in
spectrofluorometers - 3) lasers not as common but can be very useful
27
Fluorometer and Spectrofluorometer components
- Wavelength selectors filters and monochromators
(new - polychromators) - Transducers PMTs, PDAs, and CCDs or CIDs
- Cells Pyrex or quartz, no fingerprints
- Cell compartments flat black, baffles to reduce
scattered light
28
Instrument Designs - Fluorometer
Figure 15-6
29
Instrument Designs - Spectrofluorometer
Figure 15-7
30
Instrument Designs Spectrofluorometer with
Array Detector
Figure 15-8
31
Instrument Designs Spectrofluorometer with
Array Detector
Figure 15-8
32
Phosphorescence
Very similar instrumentation to fluorescence
except 1) time delay between excitation and
measurement of phosphorescence so no
fluorescence is seen 2) sample measured at
liquid N2 temperature (-196 oC) to minimize
collisional deactivation
Figure 15-3
From Skoog, Holler, and Crouch, Principles of
Instrumental Analysis, 6th ed., p. 417, Thomson
Brooks/Cole, Belmont, CA, 2007.
33
Chemiluminescence
- production of light from chemical reaction
- simplest reaction sequence
- A B C D
- C C hn
- Instrumentation?
34
Fluorometric Determination of H2O2 in Water
- This method to determine H2O2 is based on
reaction of scopoletin, a highly fluorescent
molecule, with H2O2 to produce a non fluorescent
product. - scopoletin H2O2 non
fluorescing product - lex 365 nm lem 490 nm
- Important aspect of method is that reaction is
extremely slow unless it is catalyzed. - Horse radish peroxidase is added to catalyze
reaction.
35
Fluorometric Determination of H2O2 in Water
- This method combines some of the best aspects of
external calibration curve analysis and standard
addition analysis. - Blank measurement is made using the sample, so
matrix effects are included in blank measurement. - A scopoletin solution is added to a H2O2 sample
to begin analysis. Because reaction is so slow,
no reaction takes place while first fluorescence
reading is made. - Matrix effects are taken into account because
this first reading (and all others) comes from
scopoletin in the analyte solution. - After first reading is taken, peroxidase is
added. Scopoletin and H2O2 react immediately
causing a decrease in fluorescence signal. Size
of decrease ? amount of H2O2 in sample.
36
Fluorometric Determination of H2O2 in Water
- Calibration curve is constructed by adding known
amounts of H2O2 to analyte solution and measuring
further decreases in fluorescence until a
cumulative change larger than that from original
reaction is achieved. - Cumulative changes are plotted versus cumulative
concentration of added H2O2. - Concentration of H2O2 that caused D fluorescence
from initial reaction of scopoletin and H2O2 is
then determined from calibration curve.
37
Fluorometric Determination of H2O2 in Water
- Use 3.00 mL of sample. Add 100.0 mL of pH7.0
phosphate buffer. This is solution A. - Zero instrument using solution A.
- Add 250 mL of scopoletin solution to solution A.
This is solution B. - Set instrument at maximum signal and record
reading as Finitial - Add 20 mL of peroxidase solution to solution B.
This is solution C. - Record reading of solution C as Ffinal
- Finitial - Ffinal DFsample ? H2O2 concentration
38
H2O2 analysis of rainwater
39
H2O2 analysis of rainwater
40
Concentration and Error for H2O2 analysis
7.952 0.073 mM H2O2 in 3370 mL What is H2O2
concentration, sc, and RSD in original 3.00 mL
rainwater sample?