CATALYSTS - PowerPoint PPT Presentation
1 / 10
Title: CATALYSTS
1
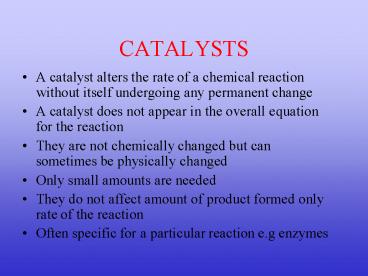
CATALYSTS
- A catalyst alters the rate of a chemical reaction
without itself undergoing any permanent change - A catalyst does not appear in the overall
equation for the reaction - They are not chemically changed but can sometimes
be physically changed - Only small amounts are needed
- They do not affect amount of product formed only
rate of the reaction - Often specific for a particular reaction e.g
enzymes
2
TYPES OF CATALYSTS
3
HOW DO HETEROGENEOUS CATALYSTS WORK?
Reactants form bonds with atoms on the surface of
the catalyst (absorbed onto the surface)
As a result bonds in the reactants are weakened
and break
4
New bonds form between the reactants held close
together on catalyst surface
This in turn weakens bonds between product and
catalyst and product leaves
5
Examples of uses of catalysts
- Car exhaust systems
- Zeolites are used in industry as catalysts e.g
in the cracking of petroleum fuels - Transition metals are good catalysts for industry
e.g iron in the Haber process
6
CATALYTIC CONVERTERS
- Catalytic converters remove harmful gases from
car exhausts. - It consists of a honeycomb of ceramic with metals
such as platinum,palladium and rhodium coated on
the honeycomb - It removes up to 90 of the harmful gases
Catalytic converter
CO Nox C8H18
CO2 N2 H2O
7
EQUATIONS FOR REACTIONS IN THE CATALYTIC CONVERTER
2
2
2
25
8
12 1/2
9
8
Catalyst poisoning
Catalysts can be poisoned. In heterogeneous
catalysis the poison molecules are absorbed
more strongly to the catalyst surface than the
reactant molecules, the catalyst becomes inactive
9
EXAMPLES OF POISONING OF CATALYSTS
Leaded petrol cannot be used in cars fitted with
a catalytic converter since lead strongly absorbs
onto the surface of the catalyst
Cannot use copper or nickel in a catalytic
converter on a car instead of the expensive
platinum or Rhodium. REASON - Any SO2 present in
the exhaust fumes (trace amounts ) would poison
the catalyst Once the catalytic converter has
become inactive it cannot be regenerated
10
REGENERATION OF CATALYST
- This is possible sometimes.
- Catalytic cracking of long-chain hydrocarbons
produces carbon - This can coat the zeolite catalyst, making it
become inactive . - The catalyst is recycled through a container
where hot air is blown over it. - Oxygen in the air converts the carbon to carbon
dioxide and the catalyst is regenerated.