Chapter 15' Conjugated Systems - PowerPoint PPT Presentation
1 / 38
Title:
Chapter 15' Conjugated Systems
Description:
1. Conjugated versus nonconjugated: Chapter 15. 3. There are ... 1. Conjugation of C=C bonds, allyl cation and radical. 2. 1,4 and 1,2 addition of H-X and X2 ... – PowerPoint PPT presentation
Number of Views:146
Avg rating:3.0/5.0
Title: Chapter 15' Conjugated Systems
1
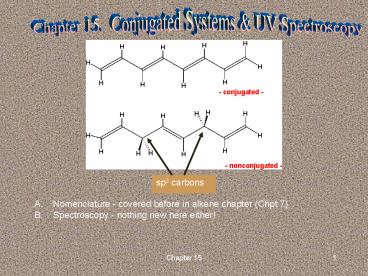
Chapter 15. Conjugated Systems UV Spectroscopy
sp3 carbons
- Nomenclature - covered before in alkene chapter
(Chpt 7) - Spectroscopy - nothing new here either!
2
C. Structure and stability 1. Conjugated versus
nonconjugated
3
There are also cummulenes
4
2. Stability issues
5
conjugation gives greater stability
6
3. Structure
7
NOTE We are skipping sections 3, 8, 9, 12 and
13B!!!!
D. 1,2 versus 1,4 addition reactions 1. addition
of HCl and HBr a. overall reaction
1, 2 addition
1,4 addition
b. mechanism (for HCl)
8
(No Transcript)
9
2. Addition of X2
10
3. Kinetic versus equilibrium control - a
return to the butadiene HBr reaction
WHY????
11
In general we have a situation where two products
can form
Normally we will have
So the yield for C2 is always greater than that
for C1 the most stable product is the one
with the lower activation barrier
12
BUT - suppose we have this
Now the most stable product (C2) has the higher
activation barrier. So what happens now????
13
- kinetic control - supply just enough energy to
get the - molecules over the activation barrier
Then the yield for C1 will be larger than that
for C2 ( because DE1 lt DE1)
14
ii. equilibrium (thermodynamic) control - supply
more than enough energy to get the molecules
over the activation barrier - get to equilbrium
for forward and back reactions.
Then the yield for C2 will be larger than that
for C1 ( because DH2 lt DH1 ie reaction 2
is more exothermic than reaction 1 C2 is more
stable than C1)
15
Br-
16
E. Allylic radicals - we have seen this before
(last semester)
17
F. The Diels-Alder reaction 1928 Diels
Alder 1. general reaction
dieneophile
diene
This reaction is great it takes two organic
molecules and combines them into one it has
makes products with precise stereochemical
control the energetics for it are right
18
2. Examples - normally the diene has no
substituents or electron donating ones (e.g.
RO-, alkyl groups, etc.). The dieneophile
normally has electron withdrawing substituents
(-CN, -C(O)R, etc.)
diene must be in cisoid conf.!
19
(No Transcript)
20
3. Mechanism - everything happens in one step
21
(No Transcript)
22
So the stereochemical consequences of the
mechanism are
23
(No Transcript)
24
4. The endo rule
25
(No Transcript)
26
5. Unsymmetrical couplings
27
(No Transcript)
28
Go home tonight and before you go to bed
29
G. UV Spectroscopy 1. experimental setup
30
E hn and n c/l where h Plancks
constant so
c speed of light E ch/l
31
(No Transcript)
32
Example
Woodward - Fieser rules Dont memorize
33
(No Transcript)
34
2. Light in action - sight!!
Trans retinal released generates a nerve impulse
35
3. Suntanning
dark - absorbs UV light in UV-A region (315 -
400nm)
36
UV-B light (290 - 315nm)
37
UV-C region (180 - 290 nm)
Ozone absorbs
UV -B and UV-C region cause skin cancer - produce
free radicals
38
H. Summary 1. Conjugation of CC bonds, allyl
cation and radical 2. 1,4 and 1,2 addition of
H-X and X2 3. Kinetic versus equilibrium control
- theory 4. NBS bromination of allylic
postions 5. Diels-Alder reaction a.
mechanism b. stereochemistry, endo rule c.
plenty of examples 6. UV spectroscopy