Coordination Compounds - PowerPoint PPT Presentation
1 / 15
Title:
Coordination Compounds
Description:
Almost all metal ions found as ... Lewis Base: electron-pair donor (has lone pair) ... Order alphabetically (by ligand, not prefix) Exceptions: Nomenclature ... – PowerPoint PPT presentation
Number of Views:850
Avg rating:3.0/5.0
Title: Coordination Compounds
1
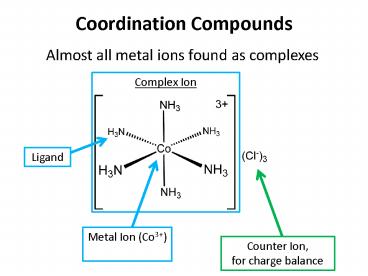
Coordination Compounds
Almost all metal ions found as complexes
Complex Ion
Ligand
Metal Ion (Co3)
Counter Ion, for charge balance
2
Ligands
- Ligand a lewis base that binds to a metal ion
- Remember
- Lewis Acid electron-pair acceptor
- Lewis Base electron-pair donor (has lone pair)
- Ligand binding results in a coordinate covalent
bond
3
Some Common Ligands
- Monodentate (one bond to metal ion)
19
4
Some Common Ligands
- Bidentate Ligands (two bonds to metal ion)
- Any ligand with more than one bond called a
chelating ligand
5
Some Common Ligands
- Polydentate Ligands
- Tridentate (3 bonds)
- Tetradentate (4 bonds)
- EDTA (ethylene diamine tetra acetate)
6
Pay attention to
- what part is the complex ion, what is the counter
ion ? - charge of the complex ion ?
- oxidation state of transition metal ?
- are ligands charged or neutral ?
Complex Ion
Transition Metal Coordination Compound
7
Determining Charge of Compound
- Necessary for Naming
- Ligands
- Some negatively charged
- Some neutral
- Metal Ion
- Always positively charged!
- Counter Ions
- Positive or Negative to balance complex ion
8
Complex Ion
If Mn is 2
- Complex Ion Charge
- Mn(H2O)6
- Mn(H2O)5Cl
- Mn(H2O)4Cl2
- MnCl6
- 2
- 1
- 0
- 4
- Ligands
- H2O
- NH3
- Cl
- OH
9
Transiton Metal Coordination Compound
- If Mn is 2
- Compound
- Mn(H2O)6
- Mn(H2O)5Cl
- Mn(H2O)4Cl2
- MnCl6
Cl2 Cl
K4
10
Charges on Vanadium?
- V (NH3)6 Cl3
- V (H2O)4 (OH)2 Cl3
- K3 V Cl6
- K V Cl3 (NH3)3
3 5 3 2
- Ligands
- H2O
- NH3
- Cl
- OH
11
Naming t.m. coordination compound
Co(NH3)5ClCl2 penta amine chloro cobalt(III)
chloride K3Fe(CN)6 potassium hexacyanoferrate
(III)
- cation is named before anion
- ligands are named before metal ion
- in naming ligands, an o is added to the root name
of an anion (e.g. fluoro, bromo). For a neutral
ligand, the name of the molecule is used, with
exception of H2O, NH3, CO and NO. - mono, di, tri, tetra, penta and hexa are used to
denote the no. of simple ligands. - bis, tris, tetrakis are used for more complicated
ligands. - oxidation state of central metal ion by a roman
number. - for gt1 type of ligand, ligands named in
alphabetical order - if the complex ion has negative charge, -ate
(suffiz) is added to the name of the metal.
12
Nomenclature
- Complex Ions ( prefix ligand )repeat
metal (charge)
Multiple Ligands Order alphabetically (by
ligand, not prefix)
Exceptions
Examples Mn Br6 1 V (H2O)4 (OH)2 3
13
Nomenclature
- Complex Ions ( prefix ligand )repeat
metal (charge)
Example Fe (NH3)6 3
Examples Pt Cl4 2 Fe Cl6 3
14
Complete Names
- cation anion
- simple anions add ide (e.g. chloride)
- or use name of polyatomic ion
- Examples
- Fe (NH3)6 Cl3
- Mn Br6 NO3
- Ca Pt Cl4
15
Bidentate Ligands
- Name as you would monodentate ligands
- Except, different prefixes
- (none)
- bis
- tris
- tetrakis
- Examples
- Co(en)(H2O)4Cl2
- Co(en)2(H2O)2Cl2