How to Interpret Titration Curves PowerPoint PPT Presentation
1 / 15
Title: How to Interpret Titration Curves
1
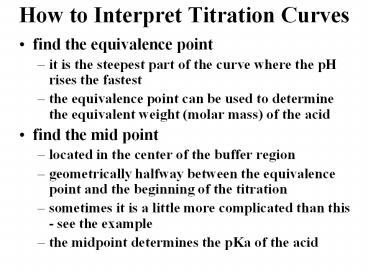
How to Interpret Titration Curves
- find the equivalence point
- it is the steepest part of the curve where the pH
rises the fastest - the equivalence point can be used to determine
the equivalent weight (molar mass) of the acid - find the mid point
- located in the center of the buffer region
- geometrically halfway between the equivalence
point and the beginning of the titration - sometimes it is a little more complicated than
this - see the example - the midpoint determines the pKa of the acid
2
How to Interpret Titration Curves
- things to do first
- graph your data as seen in the next slide
- make sure you turn on the major and minor tick
marks on both axes - right click on the axis, choose Format Axis,
change Major/Minor Tick Mark settings - Is there enough precision in the tick marks?
- you should have at least 1 mL or smaller for the
minor tick mark on the x-axis - you should have at least 0.2 pH units or smaller
for the minor tick mark on the y-axis - Format Axis, change Major/Minor Units
3
Two Different Methods
- there are two methods of analysis that will be
shown - geometric method
- requires a ruler, a pencil, and the titration
graph - 1st derivative method
- requires a spreadsheet and some formula entries
- gives you cool graphs with the 1st derivative
pointing to the equivalence and mid points - scores you brownie points with the instructors
- pick your method (either will work)
4
A Typical Titration Curve
mid point
equivalence point
5
Find the Equivalence Point (Geometric method)
1) using a ruler, draw lines that follow the
flat, more horizontal part of the curve
equivalence point
2) draw a line that follows the flat, more
vertical part of the curve
6
Find the Equivalence Point (Geometric method)
3) using a ruler, measure the distance between
the top intersection and the bottom intersection
equivalence point
4) the geometric center of this line segment is
the equivalence point
7
Find the Equivalence Point (Geometric method)
5) draw a vertical line from the equivalence
point to the x-axis
equivalence point
6) where the line crosses the x-axis is the
volume at the equivalence point (28.7 mL in this
case)
8
Find the Mid Point (Geometric method)
1) if there is a steep rise in the pH at the
beginning of the graph, draw a line that follows
the steep part of the curve
mid point
9
Find the Mid Point (Geometric method)
2) using a ruler, measure the distance between
the far left and right intersections
equivalence point
3) the geometric center between these points is
the mid point
mid point
10
Find the Mid Point (Geometric method)
4) draw a horizontal line from the mid point to
the y-axis
equivalence point
5) where the line crosses the y-axis is the pH at
the equivalence point (pH 7.2 in this case)
mid point
11
How to Interpret Titration Curves
- find the equivalence point
- make sure you subtract the initial buret volume!
- in this case, the initial buret volume was 1.07
mL - true equiv. pt. 28.7 mL - 1.07 mL 27.63 mL
- the 3 is the indicate the limit of the
significant figures - calculate the equivalent weight (molar mass)
- equiv. wt. (acid mass)/(NaOH conc)(equiv.
pt.) - equiv. wt. (430.2 mg)/(0.1139 M)(27.63mL)
- equiv. wt. 136.699 137 g/mol
12
How to Interpret Titration Curves
- find the mid point
- mid pt 7.2 pKa of the acid
13
For you Excel Aficionados
- equivalence point
- use the first derivative ? d pH / d Vol
- plot volume as x and 1st derivative as y in a 2nd
series on graph - the spike in the graph points to the equiv. pt.
- mid point
- make a new graph and reverse the axes for the pH
curve - x axis pH values y-axis Vol values
- use the first derivative ? d Vol / d pH
- plot pH as x and 1st derivative as y in a 2nd
series on graph - the spike in the graph points to the mid point
- use extra columns in the spreadsheet to make
these calcs - equiv. pt. deriv. (d pH / d Vol) (pH2 -
pH1)/(Vol2 - Vol1) - midpoint deriv. (d Vol / d pH) (Vol2 - Vol1)/
(pH2 - pH1) - or just the reciprocal of the equiv. pt.
derivative
14
Find the Equivalence Point (derivative method)
1) identify volume value at the peak
1) identify volume value at the peak (28.5 mL in
this case)
15
Find the Mid Point (derivative method)
1) identify pH value at the peak (pH 7.3 in
this case)