Nomenclature - PowerPoint PPT Presentation
1 / 17
Title: Nomenclature
1
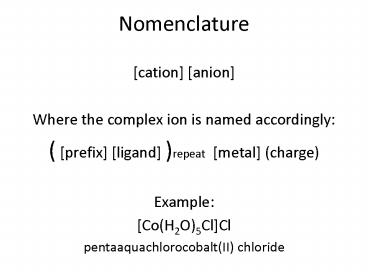
Nomenclature
- cation anion
- Where the complex ion is named accordingly
- ( prefix ligand )repeat metal (charge)
- Example
- Co(H2O)5ClCl
- pentaaquachlorocobalt(II) chloride
2
Bidentate Ligands
- Name as you would monodentate ligands
- Except, different prefixes
- (none)
- bis
- tris
- tetrakis
- Examples
- Co(en)(H2O)4Cl2
- Co(en)2(H2O)2Cl2
3
19.4 Isomerism
Isomers Same formula, different structures (and
properties)
Structural Isomers Same atoms, different bonds
Stereoisomers Same bonds, different arrangements
Coordination Isomers
Linkage Isomers
Geometric Isomers
Optical Isomers
4
Isomers
Structural Isomers Same atoms, different bonds
Coordination Isomers Swap ligand and counter ion
Linkage Isomers Same ligand, different bond to
metal
5
Coordination Isomers
Same atoms, but counter ion and ligand switched
6
Linkage Isomers
thiocyanato
isothiocyanato
Same atoms in ligand, but bound to metal through
a different atom. Often named differently.
7
Isomers
Stereoisomers Same bonds, different arrangements
Optical Isomers Different mirror
images (enantiomers)
Geometric Isomers Different positions around
metal (cis and trans)
8
Geometric Isomers
- cis (next to)
- Trans (across from)
Fig 19.11 pg 952
9
Geometric Isomers
- trans
cis
10
Optical Isomers
- These molecules also called chiral
- Chiral molecules have important properties
- Rotate plane polarized light
- Have a different 3-D shape
- Many biological process only work with one
specific optical isomer - Chiral Molecules have a non-superimposable mirror
image
11
Optical Isomers
- Rotating plane polarized light fig 19.14 pg
954
12
Determining Optical Isomers
- Nonsuperimposable mirror images
- Example Your L and R hands are optical isomers
- The mirror image of R hand can superimpose on L
hand, but not on R hand
13
Determining Optical Isomers
- Reflect molecule
Not optical isomers!
- If the reflection can not be rotated to
superimpose on original, these are optical
isomers.
14
Determining Optical Isomers
- Example
Rotate this 90
Optical isomers!
15
Stereoisomers
16
Wordage
- One structure is chiral
- The cis vs. trans molecules are geometric isomers
of each other - Two different forms of the cis version are
enantiomers of each other
17
Summary Question
- Which could display coordination, linkage,
geometrical, and optical isomerism? - a) Cr(en)(NH3)4Cl2 b)
Cr(en)2(H2O)2Cl2 c) Cr(NH3)5(SCN)Cl
d) Cr(NH3)4B2Cl2
Coordination They all could Linkage
c Geometrical b and d Optical the cis
isomer of b