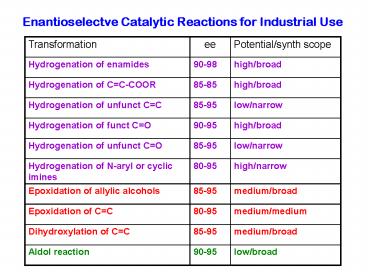
Enantioselectve Catalytic Reactions for Industrial Use - PowerPoint PPT Presentation
1 / 83
Title:
Enantioselectve Catalytic Reactions for Industrial Use
Description:
The differentiation remains in that rhodium asymmetric hydrogenation is a ... For rhodium, there are many successful ... Benzoin Condensation and Stetter Reaction ... – PowerPoint PPT presentation
Number of Views:184
Avg rating:3.0/5.0
Title: Enantioselectve Catalytic Reactions for Industrial Use
1
Enantioselectve Catalytic Reactions for
Industrial Use
2
(No Transcript)
3
The differentiation remains in that rhodium
asymmetric hydrogenation is a remarkably mild and
specific method for dehydroamino acids together
with a limited range of closely related alkenes
(e.g. itaconates) while ruthenium asymmetric
hydrogenation has a wider compass. For rhodium,
there are many successful ligands and the
opportunity for fine tuning in specific cases,
while BINAP and its close relatives have
prevailed in ruthenium asymmetric hydrogenation.
4
(No Transcript)
5
Desymmetrisation of the alkene and hydrolysis of
PGs give an enantiomerically pure drug used in
treating Parkinson's disease.
6
(No Transcript)
7
Major isomer in solution
Minor isomer (lt5)
8
(No Transcript)
9
(No Transcript)
10
(No Transcript)
11
Chiral "un-recognition" atoms clashing here
12
Chiral Recognition no clash here
13
(No Transcript)
14
(No Transcript)
15
(No Transcript)
16
(No Transcript)
17
(No Transcript)
18
Reduction of g-ketoesters
19
(No Transcript)
20
(No Transcript)
21
(No Transcript)
22
Asymmetric hydrogenation of simple ketones
When 45 atm of hydrogen is applied, 601 g of
acetophenone can be hydrogenated quantitatively
with only 2.2 mg of the Ru complex. The turnover
number is 2,400,000, while the TOF at 30
conversion is 228,000 per h or 63 per s. The
catalyst system is among the most
reactive (pre)catalyst so far reported
23
Transfer hydrogenation
24
Mechanism of Transfer Hydrogenation
- Hydrogen delivery from RuH take place by a
pericyclic mechanism via a six-membered
transition structure. - Neither carbonyl oxygen nor alcoholic oxygen
interacts with Ru throughout the hydrogen
transfer. - The carbonyl oxygen atom interacts with NH on Ru
via hydrogen bonding.
25
(No Transcript)
26
(No Transcript)
27
(No Transcript)
28
HAZARD!!
chemoselective
The alkyl group on boron can be varied. Me and
n-Bu are the common choices.
29
(No Transcript)
30
Large group equatorial
31
(No Transcript)
32
(No Transcript)
33
(No Transcript)
34
(No Transcript)
35
(No Transcript)
36
(No Transcript)
37
(No Transcript)
38
(No Transcript)
39
(No Transcript)
40
Sometimes, control of enolate geometry is
unnecessary.
In open chain carbonyl compounds, control is
necessary.
41
(No Transcript)
42
We need access to both geometries of enolates.
By contrast,
43
(No Transcript)
44
(No Transcript)
45
Asymetric aldol reactions
46
(No Transcript)
47
(No Transcript)
48
Boron chelation controls enolate geometry, but
boron cannot have gt4 ligands, so chelation must
change for the aldol reaction to occur.
49
(No Transcript)
50
(No Transcript)
51
(No Transcript)
52
(No Transcript)
53
(No Transcript)
54
(No Transcript)
55
(No Transcript)
56
(No Transcript)
57
(No Transcript)
58
Catalytic Aldol Reaction with cyclic transition
state
S.E. Denmark
59
Stereoselective synthesis of Z and E silyl enol
ethers
60
Evans' Chiral Auxiliary in a Different
Context Diastereoselective Diels-Alder
Cycloadditions
61
(No Transcript)
62
(No Transcript)
63
(No Transcript)
64
(No Transcript)
65
(No Transcript)
66
(No Transcript)
67
(No Transcript)
68
(No Transcript)
69
Introduction to Organocatalysis
70
(No Transcript)
71
(No Transcript)
72
Cycloaddition
73
(No Transcript)
74
Synthetic application of organocatalytic
cycloaddition
75
Benzoin Condensation and Stetter Reaction
76
(No Transcript)
77
(No Transcript)
78
Acyl Transfer Kinetic Resolution and
Desymmetrisation
79
(No Transcript)
80
(No Transcript)
81
(No Transcript)
82
(No Transcript)
83
(No Transcript)