Naming Ionic Compounds with Multivalent Metals PowerPoint PPT Presentation
Title: Naming Ionic Compounds with Multivalent Metals
1
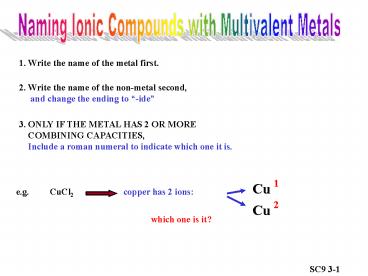
Naming Ionic Compounds with Multivalent Metals
1. Write the name of the metal first.
2. Write the name of the non-metal second,
and change the ending to -ide
3. ONLY IF THE METAL HAS 2 OR MORE COMBINING
CAPACITIES, Include a roman numeral to
indicate which one it is.
1
Cu
e.g.
CuCl2
copper has 2 ions
2
Cu
which one is it?
SC9 3-1
2
Predicting Ion Charges from Formulas
To find out which copper ion it is, work out the
ions by criss-crossing in reverse.
CuCl2
Cu Cl
2
1
Check the combining capacity of the non-metal to
make sure that it is correct.
2
1
Cu
Cl
Therefore it is copper (II) chloride
SC9 3-2
3
More Examples
FeO
FeS2
Fe O
Fe S
1
1
2
1
1
1
2
1
Fe
O
Fe
S
Check
Check
FeO Iron (II) oxide
FeS2 Iron (IV) sulphide
SC9 3-3
4
Practice
Name each of the following compounds
a) CuO
Copper (II) oxide
Combining Capacities O 2 As 3 Br 1 Cl 1
b) FeAs
Iron (III) arsenide
c) CrBr3
Chromium (III) bromide
d) Cu2O
Copper (I) oxide
e) FeCl3
Iron (III) chloride
f) CrCl2
Chromium (II) chloride
SC9 3-4
PowerShow.com is a leading presentation sharing website. It has millions of presentations already uploaded and available with 1,000s more being uploaded by its users every day. Whatever your area of interest, here you’ll be able to find and view presentations you’ll love and possibly download. And, best of all, it is completely free and easy to use.
You might even have a presentation you’d like to share with others. If so, just upload it to PowerShow.com. We’ll convert it to an HTML5 slideshow that includes all the media types you’ve already added: audio, video, music, pictures, animations and transition effects. Then you can share it with your target audience as well as PowerShow.com’s millions of monthly visitors. And, again, it’s all free.
About the Developers
PowerShow.com is brought to you by CrystalGraphics, the award-winning developer and market-leading publisher of rich-media enhancement products for presentations. Our product offerings include millions of PowerPoint templates, diagrams, animated 3D characters and more.