Avastin bevacizumab in metastatic breast cancer MBC - PowerPoint PPT Presentation
1 / 11
Title:
Avastin bevacizumab in metastatic breast cancer MBC
Description:
AVADO PFS Analysis (ITT Population) All P values vs. placebo ... Protocol-defined; mg/kg q3w; **RPLS=reversible posterior leuko encephalopathy syndrome ... – PowerPoint PPT presentation
Number of Views:410
Avg rating:3.0/5.0
Title: Avastin bevacizumab in metastatic breast cancer MBC
1
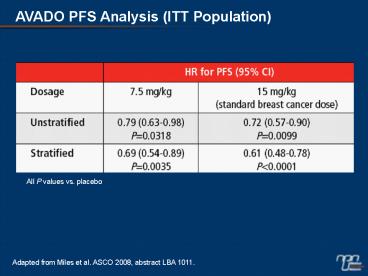
AVADO PFS Analysis (ITT Population)
All P values vs. placebo
Adapted from Miles et al. ASCO 2008, abstract LBA
1011.
2
AVADO Response (patients with measurable
disease),
mg/kg q3w
Adapted from Miles et al. ASCO 2008, abstract LBA
1011.
3
E2100 and AVADO Serious Adverse Events ()
ATE arterial thromboembolic events. No increase
in venous thromboembolic events was observed
with bevacizumab in either study
- Adapted from Miller et al. N Engl J Med
2007357-2666-76. - Adapted from Miles et al. ASCO 2008, abstract
LBA 1011.
4
E2100 Study Design Randomized, double-blind,
placebo-controlled, multicentre, phase III trial
Paclitaxel 90 mg/m2 qw for 3 weeks of a 4-week
cycle (n354)
Progressive disease (PD)
Previously untreated MBC (n722)
No crossover permitted
Paclitaxel bevacizumab 10 mg/kg q2w (n368)
PD
- Primary end point progression-free survival
(PFS) - Secondary end points overall response rate,
overall survival, quality of life
Adapted from Miller et al. N Engl J Med
20073572666-76.
5
E2100 Trial PFS Results
Paclitaxel/bevacizumab 11.4 months
1.0
Paclitaxel 6.11 months
0.8
HR0.51 (0.43-0.62) Log rank test Plt0.0001
0.6
PFS proportion
0.4
0.2
6.11
11.4
0.0
0
6
12
18
24
30
Months
484 events reported (89 of required events)
Adapted from Miller et al. N Engl J Med
20073572666-76.
6
E2100 Trial Overall Response Rate
Paclitaxel Paclitaxel/bevacizumab
Plt0.0001
Plt0.0001
40
37.7
29.9
30
Overall response rate ()
20
16.0
13.8
10
339
341
262
236
0
All patients
Measurable disease
Adapted from Miller et al. N Engl J Med
20073572666-76.
7
AVADO Study Design Randomized, double-blind,
placebo-controlled, multicentre, phase III trial
Docetaxel 100 mg/m2 q3w placebo
PD
All patients were given the option to receive
bevacizumab with second line chemotherapy
Previously untreated MBC (n705)
Docetaxel bevacizumab 7.5 mg/kg q3w
PD
Docetaxel bevacizumab 15 mg/kg q3w
PD
Docetaxel was administered for a maximum of nine
cycles but earlier discontinuation was permitted
- Primary end point PFS
- Secondary end points overall response rate,
duration of response, time to treatment failure,
overall survival, safety and quality of life
Adapted from Miles et al. ASCO 2008, abstract
LBA1011.
8
AVADO Response (patients with measurable
disease),
Adapted from Miles et al. ASCO 2008, abstract
LBA1011.
9
AVADO Safety Summary
Adapted from Miles et al. ASCO 2008, abstract
LBA1011.
10
AVADO Grade 3 Adverse Events of Special
Interest,
Adapted from Miles et al. ASCO 2008, abstract
LBA1011.
11
Ongoing RIBBON 1 Phase IIITrial Study Design
Chemotherapy bevacizumab i.v. 15mg/kg q3w or
10 mg/kg q2w
Chemotherapy bevacizumab i.v. 15mg/kg q3w
PD
Previously untreated MBC (n950), 21
Randomization
Chemotherapy placebo (i.v. on day 1 of
21-day cycle)
Chemotherapy crossover to bevacizumab 15mg/kg
q3w or 10 mg/kg q2w
PD
Anthracycline-based combination chemotherapy, Q3w
taxane (docetaxel or protein-bound paclitaxel) or
capecitabine as determined by investigator prior
to randomization Chemotherapy regimen at
investigator discretion
Primary end point hierarchical PFS
Adapted from Albain K. ASCO 2008.