Review - PowerPoint PPT Presentation
1 / 14
Title:
Review
Description:
2. Place least electronegative element. in center (never H) CH3OH. element group number e ... 2. Place least electronegative element. in center (never H) ... – PowerPoint PPT presentation
Number of Views:35
Avg rating:3.0/5.0
Title: Review
1
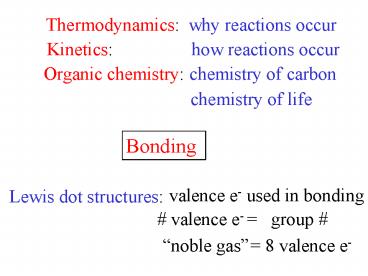
Thermodynamics
why reactions occur
Kinetics
how reactions occur
Organic chemistry
chemistry of carbon
chemistry of life
Bonding
valence e- used in bonding
Lewis dot structures
group
valence e-
noble gas
8 valence e-
2
Lewis structures
CH3OH
1. Determine valence e-
element group number e-
C
IV
4
H
I
1
O
VI
6
2. Place least electronegative element in
center (never H)
3
non-polar covalent ? 0.4
polar covalent 0.5-1.8
NaCl
3.0 - 0.9 2.1
ionic
Cl-Cl 3.0 - 3.0 0.0 covalent
C-O
3.5 - 2.5
1.0
polar covalent
C-H
2.5 - 2.1
0.4
covalent
4
Lewis structures
1. Determine valence e-
2. Place least electronegative element in
center (never H)
C
3. Make single bond (2e-) between each pair
of atoms
4. Use remaining e- to satisfy octet rule
5. Use double or triple bonds to reduce
of unshared e-
5
Lewis structures
CH3OH
C H O
valence e-
4
4
6
14 e-
H
C
O
H
H
H
6
Lewis structures
formal charge
COCl2
C O Cl
valence e-
4
6
14
24 e-
..
..
C
Cl
Cl
..
..
..
..
..
7
- 4
- 2
1
..
..
..
O
..
..
..
6 -
6
- 1
-1
7
- 6
- 1
0
..
6 -
4
- 2
0
group number
- unshared e- -
(1/2) shared e-
7
VSEPR
Molecular geometries
?-
H2O
O
H
H
?
?
pairs of valence e- on O
tetrahedron
4
bonds angles 109.5o
actually 108o
dipole moment
H-bonding
polar bonds
H bound to
O,
N,
F
H-bond donor
H-bond acceptor
8
VSEPR
Molecular geometries
?
CH2O
C
H
H
O
?-
3
trigonal planar
pairs of e- on C
bonds angles 120o
dipole moment
polar bond
dipole-dipole
9
VSEPR
Molecular geometries
?
CO2
C
O
O
?-
?-
2 bonding pairs of e- on C
linear
bonds angles 180o
no dipole moment
LDF
polar bonds
10
H2O
O
H
H
O ___ s electrons ___ p electrons
2
4 equivalent orbitals
4
Hybridization
s
p
3
?-bond
molecular orbitals
11
C
H
H
CH2O
O
C ___ s electrons ___ p electrons
2
3 equivalent orbitals
2
Hybridization
p
?-bond
sp2
?-bond
molecular orbitals
12
C
H
H
CH2O
O
O ___ s electrons ___ p electrons
2
3 equivalent orbitals
4
Hybridization
p
?-bond
sp2
?-bond
molecular orbitals
13
CO2
C
O
O
C ___ s electrons ___ p electrons
2
2 equivalent orbitals
2
Hybridization
p
?-bond
sp
?-bond
molecular orbitals
14
CO2
C
O
O
O ___ s electrons ___ p electrons
2
3 equivalent orbitals
4
Hybridization
p
?-bond
sp2
?-bond
molecular orbitals