DG DGo' RTlnQ PowerPoint PPT Presentation
1 / 28
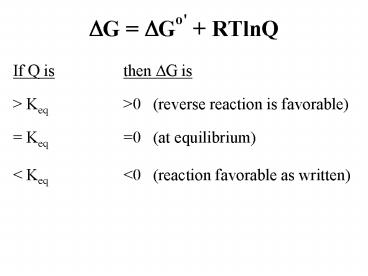
Title: DG DGo' RTlnQ
1
DG DGo' RTlnQ
- If Q is then DG is
- gt Keq gt0 (reverse reaction is favorable)
- Keq 0 (at equilibrium)
- lt Keq lt0 (reaction favorable as written)
2
Standard States in Biochemistry 1. Activity of
water is 1. (really 55 M) 2. Hydrogen ion
activity is 1 at pH 7. ?Go
3
13-2
Table 13-2 p 362 in VVP
4
Acids, Bases and Buffers!!!
- pH pKa log A
- HA
5
Bloody Fact
- If 1 mL of 10 N HCl is added to 1 liter of saline
solution at pH 7.0, the pH will decrease to
roughly pH 2. - If 1 mL of 10 N HCl is added to 1 liter of blood
plasma at pH 7.4, the pH will decrease to pH
7.2. - Why? Blood is buffered (in this case by the
H2CO3/HCO3 system).
6
This is IMPORTANT!!!
- If pH pKa, then A- HA
- then deprotonated protonated
- If pH lt pKa, then A- lt HA
- then deprotonated lt protonated
- If pH gt pKa, then A- gt HA
- then deprotonated gt protonated
Summarized on VVP Fig 2-15
7
Using Henderson-Hasselbalch
- at pH values 3 pH units from pKa the group is
essentially fully deprotonated or fully
protonated, so the average charge 0 or 1. - at pH pKa the group is 50 protonated, thus it
carries an average charge 0.5 - H-H equation can be used to calculate the average
charge on an ionizable group at any pH.
8
VVP Fig 2-15
9
VVP Fig 2-16
pHpKa3
pHpKa2
pHpKa1
10
BUILDING BLOCKS!!! NUCLEOTIDES AMINO ACIDS
11
VVP Table 4-1
0.091
X
12
amino acid structures
See Table 4-1 p80 in VVP
13
See Table 4-1 p80 in VVP
14
Amino acid structures
http//info.bio.cmu.edu/Courses/ BiochemMols/AAVie
wer/ AAVFrameset.htm
15
Ionic properties of amino acids impart ionic
properties to proteins
- in general these are SURFACE properties (i.e.
charged sidechains are on solvent-exposed outside
of folded structure) - affect protein-ligand binding (e.g. DNA-binding
proteins) or catalysis - average charge on protein is an important
consideration in the design of a purification
process
16
pKa3
pKa2
pKa1
17
Other Properties of Amino Acids
- Stereochemistry (all biosynthetic proteins made
up of L-isomer) - Hydropathy (partitioning between polar and
nonpolar solvents as indicator of polarity) (see
Table 6-2 in VVP p 150 Take Note p58) - these two properties are major determinants of
peptide conformation
18
(No Transcript)
19
See VVP Fig 4-3
20
(No Transcript)
21
VVP Fig 6-3 p 126
22
(No Transcript)
23
Example of a protein sequence
N-terminus
- MANSKINKQL DKLPENLRLN GRTPSGKLRS FVCEVCTRAF
ARQEHLKRHY - RSHTNEKPYP CGLCNRCFTR RDLLIRHAQK IDSGNLGETI
SHTKKVSRTI - TKARKNSASS VKFQTPTYGT PDNGGSGGTV LSEGEWQLVL
HVWAKVEADV - AGHGQDILIR LFKSHPETLE KFDRFKHLKT EAEMKASEDL
KKHGVTVLTA - LGAILKKKGH HEAELKPLAQ SHATKHKIPI KYLEFISEAI
IHVLHSRHPG - DFGADAQGAM NKALELFRKD IAAKYKELGY G
C-terminus
24
VVP page 150
nonpolar
polar
25
(No Transcript)
26
VVP Fig 6-1 p 125
27
VVP Fig 5-1 p 94
C-termini
N-termini
28
(Rasmol)