Calculation of Formula Weight PowerPoint PPT Presentation
1 / 39
Title: Calculation of Formula Weight
1
Calculation of Formula Weight
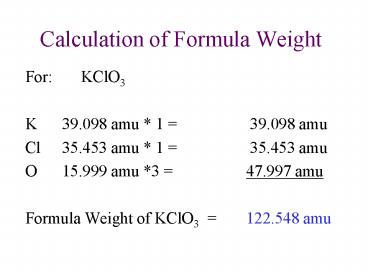
- For KClO3
- K 39.098 amu 1 39.098 amu
- Cl 35.453 amu 1 35.453 amu
- O 15.999 amu 3 47.997 amu
- Formula Weight of KClO3 122.548 amu
2
Calculation of Formula Weight
- For sucrose (sugar) the chemical formula is
C12H22O11 - C 12.011 amu
- H 1.008 amu
- O 15.999 amu
- Calculate the formula weight of sucrose to 4 sig
figs
3
Calculating Composition
- the percent composition by mass of O in H2O
- the total mass of O present in H2O
- the total mass of H2O
O in H2O
4
Using Composition by Mass
- (1) a sample of clear liquid (3.0000 g) is found
to contain 0.1778 g H and 2.8222 g O - (2) a sample of clear liquid (5.0000 g) is found
to contain 0.5595 g H and 4.4405 g O - (3) a sample of clear liquid (7.0000 g) is found
to contain 0.2946 g H and 4.6769 g O - Are these the same or different compounds?
5
(No Transcript)
6
The Mole
- The mole describes the number of particles
(atoms or molecules) in a given mass of a
substance (element or compound)
7
How many objects in 1 mole?
- 602,213,670,000,000,000,000,000 objects
- 6.022 x 1023 objects (Avogadros number)
- e.g. one mole of an element contains 6.022 x 1023
atoms one mole of compound contains 6.022 x 1023
molecules (or formula units)
8
See handout on the MOLE concept
1 dozen molecules of CH4 reacts with 2 dozen
molecules of O2 to form products 1 thousand
molecules of CH4 reacts with 2 thousand molecules
of O2 to form products 1 109 molecules of CH4
reacts with 2 109 molecules of O2 to form
products 1 6.022E23 molecules of CH4 reacts
with 2 6.022E23 molecules of O2 to form
products
9
Molar Mass
- The mole is defined as the number of atoms in
exactly 12 g of 12C. - For any element a mole of atoms is present in a
mass the value of the atomic weight for that
element in grams. This is defined as the molar
mass for the element. - For any compound a mole of molecules is present
in a mass the value of the formula weight for
that compound in grams . This is defined as the
molar mass for the compound.
10
(No Transcript)
11
What YOU need to know
- How to use the atomic weight information in the
periodic table - The average mass of one atom is given as the
atomic weight in units of amu - The mass of one mole of atoms is given as the
molar mass by replacing the unit amu with the
unit gram
12
How many moles...
- in 10.8 g of boron?
- in 25.0 g of boron?
- in 342.3 g of sucrose?
- in 10.0 g of sucrose?
- of O in 1.0 mole of sucrose?
- of O in 2.30 mole of sucrose?
- of H in 54 g of water?
13
Chemical Equations
- identify all reactants and products
- shows molar ratios between all chemical species
in the reaction (stoichiometry) - proper stoichiometry is represented ONLY when the
chemical equation is balanced
14
(No Transcript)
15
Using Stoichiometry
- Stoichiometry is the basis for answering two
fundamental questions in chemical analysis - What is the theoretical yield?
- What is the limiting reagent?
- REMEMBER stoichiometry shows molar ratios not
mass ratios
16
When 66.6 g of O2 gas is mixed with 27.8 g of NH3
gas and 25.1 g of CH4 gas, 36.4 g of HCN gas is
produced by the following reaction 2CH4 2NH3
3O2 2HCN 6H2O Given 17.03 g
NH3/mol, 32.00 g O2/mol, 27.03 g HCN/mol, 16.04 g
CH4/mol What is the percent yield of HCN in this
reaction?
17
Percent yield
- yield actual yield
- actual yield is the observed yield of product
- theoretical yield is calculated assuming 100
conversion of the LIMITING REAGENT
100
theoretical yield
18
Limiting Reagent
- The limiting reagent is the reactant in a
chemical reaction which limits the amount of
products that can be formed. - The limiting reagent in a chemical reaction is
present in insufficient quantity to consume the
other reactant(s). - This situation arises when reactants are mixed in
non-stoichiometric ratios.
19
See Figure 3.14
20
Figure 3.15
21
(No Transcript)
22
Theoretical Yield Which Reactant is Limiting?
- 1) calculate moles (or mass) of product formed by
complete reaction of each reactant. - 2) the reactant that yields the least product is
the limiting reagent. - 3) the theoretical yield for a reaction is the
maximum amount of product that could be generated
by complete consumption of the limiting reagent.
23
66.6 g of O2 2.08 mol O2 27.8 g of NH3 1.63
mol NH3 25.1 g of CH4 1.56 mol CH4 Which
reactant is limiting? 2.08 mol O2 can yield 1.39
mol (or 37.5 g) HCN 1.63 mol NH3 can yield 1.63
mol (or 44.1 g) HCN 1.56 mol CH4 can yield 1.56
mol (or 42.2 g) HCN
24
O2 is the limiting reagent thus, the theoretical
yield is based on 100 consumption of O2. 2.08
mol O2 can yield 1.39 mol (or 37.5 g) HCN
- yield actual yield
100
theoretical yield
yield 36.4 g HCN
97.1
100
37.5 g HCN
25
When 66.6 g of O2 gas is mixed with 27.8 g of NH3
gas and 25.1 g of CH4 gas, 36.4 g of HCN gas is
produced by the following reaction 2CH4 2NH3
3O2 2HCN 6H2O Given 17.03 g
NH3/mol, 32.00 g O2/mol, 27.03 g HCN/mol, 16.04 g
CH4/mol What is the percent yield of HCN in this
reaction? How many grams of NH3 remain?
26
2CH4 2NH3 3O2 2HCN 6H2O How many
grams of NH3 remain? 36.4 g (or 1.35 mol) of HCN
gas is produced Since the reaction stoichiometry
is 11, 1.35 mol of NH3 is consumed (1.63 mol
NH3 initially) (1.35 mol NH3 consumed) 0.28
mol NH3 remaining 0.28 mol NH3 (17.03 NH3
g/mol NH3) 4.8 g NH3 remain
27
(No Transcript)
28
(No Transcript)
29
Elemental analysis C and H mass by
weight (C,H,O) xs O2 CO2
H2O The mass of CO2 recovered gives the mass of
C in the sample and the mass of H2O recovered
gives the mass of H in the sample.
Figure 3.6
30
What is the empirical formula for the following
unknown sample?
- Sample is carbohydrate (i.e., composed of C, H,
O). - 50.00 g of sample yields
- 73.29 g CO2
- 30.00 g H2O
- Molar mass of CO2 44.01 g/mol
- Molar mass of H2O 18.02 g/mol
31
Calculating Empirical Formula from
compositiona mnemonic
- 1) to mass
- 2) mass to mole
- 3) divide by small
- 4) multiply til whole
- step 1 does composition change as a function
of amount of material?
32
composition of H2O by mass
- 88.81 O 11.19 H
- 5 g H2O is 4.441 g O and 0.559 g H by mass
- 45 g H2O is 39.965 g O and 5.035 g H by mass
- 50 g H2O is 44.405 g O and 5.595 g H by mass
- 100 g H2O is 88.81 g O and 11.19 g H by mass
33
Calculating an Empirical Formula
mass of each element in the sample
- composition
moles of each element in the sample
use of moles as subscript in formula
multiply by integer such that all subscripts are
whole numbers
divide all subscripts by smallest subscript
34
What is the empirical formula for the following
unknown sample?
- Sample is carbohydrate (i.e., composed of C, H,
O). - 50.00 g of sample yields
- 73.29 g CO2
- 30.00 g H2O
- Molar mass of CO2 44.01 g/mol
- Molar mass of H2O 18.02 g/mol
35
Empirical vs. Molecular Formula
n (empirical formula) molecular formula
- Compound EF MF n
- formaldehyde CH2O CH2O 1
- acetic acid CH2O C2H4O2 2
- glucose CH2O C6H12O6 6
- All 3 compounds are 40.00 C
- 6.714 H
- 53.27 O
36
(No Transcript)
37
To determine the molecular formula we need
information about the mass of a molecule Mass
Spectrometry
38
What is the empirical formula for the following
unknown sample?
- Elemental analysis shows
- 75.40 C
- 4.43 H
- 20.10 O
39
Example Problem 3.63 page 119
Combustion of 6.38 mg of ethylene glycol gives
9.06 mg CO2 and 5.58 mg H2O. The compound only
contains C, H, and O. What are the mass
percentages of the elements in ethylene glycol?