Writing Lewis Structures for Molecular (Covalent) Compounds PowerPoint PPT Presentation
Title: Writing Lewis Structures for Molecular (Covalent) Compounds
1
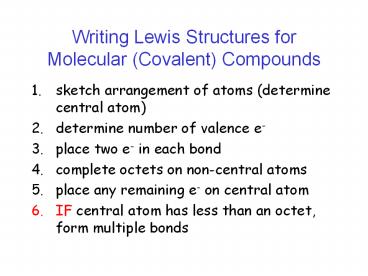
Writing Lewis Structures for Molecular (Covalent)
Compounds
- sketch arrangement of atoms (determine central
atom) - determine number of valence e-
- place two e- in each bond
- complete octets on non-central atoms
- place any remaining e- on central atom
- IF central atom has less than an octet, form
multiple bonds
2
Drawing Lewis Structures
3
Draw the Lewis structure for the NO21- ion.
First, determine the atomic arrangement.
4
Next, determine the total number of
valence electrons.
5 electrons from the N
6 electrons from each O for 12 more electrons
1 additional electron because it is a monoanion
5
Use the electrons to make electron-pair bonds
between the central and outer atoms.
18 - 4 14 electrons remain
6
Complete the octet of each outer atom.
14 - 12 2 electrons remaining
7
If there are any remaining electrons, put them on
the central atom.
..
All of the valence electrons have now been used.
8
If, and only if, the central atom has fewer than
eight electrons, complete its octet by using
nonbonding electron pairs from outer atoms to
make multiple bonds.
9
In the final Lewis structure, each atom should
have a complete octet of electrons. If it is an
ion, indicate the charge outside of brackets.
PowerShow.com is a leading presentation sharing website. It has millions of presentations already uploaded and available with 1,000s more being uploaded by its users every day. Whatever your area of interest, here you’ll be able to find and view presentations you’ll love and possibly download. And, best of all, it is completely free and easy to use.
You might even have a presentation you’d like to share with others. If so, just upload it to PowerShow.com. We’ll convert it to an HTML5 slideshow that includes all the media types you’ve already added: audio, video, music, pictures, animations and transition effects. Then you can share it with your target audience as well as PowerShow.com’s millions of monthly visitors. And, again, it’s all free.
About the Developers
PowerShow.com is brought to you by CrystalGraphics, the award-winning developer and market-leading publisher of rich-media enhancement products for presentations. Our product offerings include millions of PowerPoint templates, diagrams, animated 3D characters and more.