Introduction to Analytical Separations - PowerPoint PPT Presentation
1 / 43
Title:
Introduction to Analytical Separations
Description:
A separation technique based on the different rates of travel of solutes through ... Relative Retention (a): ratio of adjusted retention time between two solutes ... – PowerPoint PPT presentation
Number of Views:951
Avg rating:5.0/5.0
Title: Introduction to Analytical Separations
1
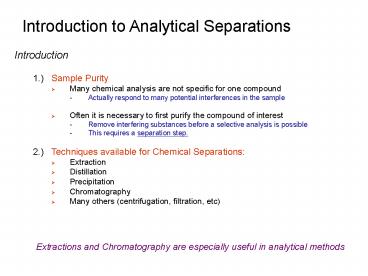
Introduction to Analytical Separations
- Introduction
- 1.) Sample Purity
- Many chemical analysis are not specific for one
compound - Actually respond to many potential interferences
in the sample - Often it is necessary to first purify the
compound of interest - Remove interfering substances before a selective
analysis is possible - This requires a separation step.
- 2.) Techniques available for Chemical
Separations - Extraction
- Distillation
- Precipitation
- Chromatography
- Many others (centrifugation, filtration, etc)
Extractions and Chromatography are especially
useful in analytical methods
2
Introduction to Analytical Separations
- Introduction
- 3.) Illustration
- Biological Samples are Composed of Complex
Mixtures - Analysis of composition and changes help in
understanding disease and the development of
treatments
NMR Spectra of Mouse Urine after treatment with a
Drug
Analysis of Various Pesticides in Ground water
using LC-MS
2D Gel Electrophoresis of total protein extract
from E. coli cells
Journal of Chromatography A, 1109 (2006) 222227
Electrophoresis (1997) 181259-1313
Toxicological Sciences (2000) 57326-337
3
Introduction to Analytical Separations
- Extractions
- 1.) Definition
- The transfer of a compound from one chemical
phase to another - The two phases used can be liquid-liquid,
liquid-solid, gas-solid, etc - Liquid-liquid is the most common type of
extraction - The partitioning of solute s between two chemical
phases (1 and 2) is described by the equilibrium
constant K
Immiscible liquids
K is called the partition coefficient
4
Introduction to Analytical Separations
- Extractions
- 2.) Extraction Efficiency
- The fraction of moles of S remaining in phase 1
after one extraction can be determined - The value of K and the volumes of phases 1 and 2
need to be known - The fraction of S remaining in phase 1 after n
extractions is
where q fraction of moles of S remaining in
phase 1 V1 volume of phase 1 V2 volume of
phase 2 K partition coefficient
Assumes V2 is constant
5
Introduction to Analytical Separations
- Extractions
- 2.) Extraction Efficiency
- Illustration
Ether layer
Water layer
1M UO2(NO3)2 (yellow)
After mixing, UO2(NO3)2 Is distributed in both
layers
After 8 extractions, UO2(NO3)2 has been removed
from water
6
Introduction to Analytical Separations
- Extractions
- 3.) What happens as n approaches infinity?
- Eventually the amount of S remaining in phase 1
becomes zero - Solution is infinitely diluted
This Situation Created a Strange Saga in Science
Water Memory
- a founding principal of homeopathic medicine
- the claim is that water remembers the activity
of the drug after it has been removed
Nature (1988) 333816-818
Authors claim to still observe antibody activity
even after a 1x10120 fold dilution. Less than 1
molecule is present with a 1x1014 dilution
A number of subsequent studies have disputed the
claim but the controversy is still popular in the
press and as alternative medicine, even though
the results are consistent with the placebo
effect.
7
Introduction to Analytical Separations
- Extractions
- 4.) Example 1
- Solute A has a K 3 for an extraction between
water (phase 1) and benzene (phase 2). - If 100 mL of a 0.01M solution of A in water is
extracted one time with 500 mL benzene, what
fraction will be extracted?
Solution
First determine fraction not extracted (fraction
still in phase 1, q)
The fraction of S extracted (p) is simply
8
Introduction to Analytical Separations
- Extractions
- 4.) Example 2
- For the same example, what fraction will be
extracted if 5 extractions with 100 mL benzene
each are used (instead of one 500 mL extraction)?
Solution
Determine fraction not extracted (fraction still
in phase 1, q)
The fraction of S extracted (p) is
Note For the same total volume of benzene (500
mL), more A is extracted if several small
portions of benzene are used rather than one
large portion
9
Introduction to Analytical Separations
- Extractions
- 5.) pH Effects in Extractions
- For weak acids (HA) and Bases (B)
- Protonated and non-protonated forms usually have
different partition coefficients (K) - Charged form (A- or BH) will not be extracted
- Neutral form (HA or B) will be extracted
- Partitioning is Described in Terms of the Total
Amount of a Substance - Individual concentrations of B BH or HA A-
are more difficult to determine - Partitioning is regardless of the form in both
phases - Described by the distribution coefficient (D)
10
Introduction to Analytical Separations
- Extractions
- 5.) pH Effects in Extractions
- The distribution of a weak base or weak acid is
pH dependent
For a weak base (B) where BH only exists in
phase 1
11
Introduction to Analytical Separations
- Extractions
- 5.) pH Effects in Extractions
- The distribution of a weak base or weak acid is
pH dependent
Substitute definition of KB and Ka into D
(equilibrium constant)
(partition coefficient)
D is directly related to H
12
Introduction to Analytical Separations
- Extractions
- 5.) pH Effects in Extractions
- A similar expression can be written for a weak
acid (HA) - The ability to change the distribution ratio of a
weak acid or weak base with pH is useful in
selecting conditions that will extract some
compounds but not others. - Use low pH to extract HA but not BH (weak acid
extractions) - Use high pH to extract B but not A- (weak base
extractions)
where
13
Introduction to Analytical Separations
- Extractions
- 6.) Example
- Butanoic acid has a partition coefficient of 3.0
(favoring benzene) when distributed between water
and benzene. Find the formal concentration of
butanoic acid in each phase when 100 mL of 0.10 M
aqueous butanoic acid is extracted with 25 mL of
benzene at pH 4.00 and pH 10.00
14
Introduction to Analytical Separations
- Extractions
- 7.) Extractions with a Metal Chelator
- Metal ions may be separated from one another by
using various organic complexing agents. - Soluble in organic solvent
15
Introduction to Analytical Separations
- Extractions
- 7.) Extractions with a Metal Chelator
- Common complexing agents
Crown ethers
16
Introduction to Analytical Separations
- Extractions
- 7.) Extractions with a Metal Chelator
- Many of the complexing agents bind to a variety
of metals - Different strengths or equilibrium constants
- A metal ion extraction may be made selective for
a particular metal by - Choosing a complexing agent a high affinity to
the metal (small K) - Adjusting the pH of the extraction
Cu2 is completely extracted at pH 5 while Zn2
remains in aqueous phase
pH selectivity of dithizone metal ion extraction
17
Introduction to Analytical Separations
- Chromatography
- 1.) Definition
- A separation technique based on the different
rates of travel of solutes through a system
composed of two phases - A stationary phase
- A mobile phase
- Detect compounds emerging in column by changes in
absorbance, voltage, current, etc
Chromatogram (not spectrum)
18
Introduction to Analytical Separations
- Chromatography
- 2.) System Components and Process
- Stationary Phase the chemical phase which
remains in the column (chromatographic system) - Mobile Phase (eluent) the chemical phase which
travels through the column - Support a solid onto which the stationary phase
is chemically attached or coated
Solute are separated in chromatography by their
different interactions with the stationary phase
and mobile phase
19
Introduction to Analytical Separations
- Chromatography
- 2.) System Components and Process
Solutes which interact more strongly with the
stationary phase take longer to pass through the
column
Strongly Retained
Weakly Retained
Solutes which only weakly interact with the
stationary phase or have no interactions with it
elute very quickly
20
Introduction to Analytical Separations
- Chromatography
- 3.) Chromatogram
- Chromatogram graph showing the detector response
as a function of elution time. - Retention time (tr) the time it takes a compound
to pass through a column - Retention volume (Vr) volume of mobile phase
needed to push solute through the column
Retention time
Non-retained solute (void volume)
The strength or degree with which a molecule is
retained on the column can be measured using
retention time or retention volume.
21
Introduction to Analytical Separations
- Chromatography
- 4.) Fundamental Measures of Solute Retention
- Adjusted retention time (tr) the additional
time required for a solute to travel through a
column beyond the time required for non-retained
solute - Relative Retention (a) ratio of adjusted
retention time between two solutes - Greater the relative retention the greater the
separation between two components
where tm minimum possible time for a
non-retained solute to pass through the column
where tr2 gt tr1 , so a gt 1
22
Introduction to Analytical Separations
- Chromatography
- 4.) Fundamental Measures of Solute Retention
- Capacity factor (k)
- The longer a component is retained by the column,
the greater the capacity factor - Capacity factor of a standard can be used to
monitor performance of a column - Capacity factor is equivalent to
23
Introduction to Analytical Separations
- Chromatography
- 4.) Fundamental Measures of Solute Retention
- Capacity factor is equivalent to
where Cs concentration of solute in the
stationary phase Cm concentration of solute in
the mobile phase Vs volume of the stationary
phase Vm volume of the mobile phase
24
Introduction to Analytical Separations
- Chromatography
- 4.) Fundamental Measures of Solute Retention
- Capacity factor is equivalent to
- Similar relationship for relative retention
Under equilibrium conditions
(partition coefficient)
Capacity factor is directly proportional to
partition coefficient
25
Introduction to Analytical Separations
- Chromatography
- 4.) Fundamental Measures of Solute Retention
- Example
The retention volume of a solute is 76.2 mL for a
column with Vm 16.6 mL and Vs 12.7 mL.
Calculate the capacity factor and the partition
coefficient for this solute.
26
Introduction to Analytical Separations
- Chromatography
- 5.) Efficiency of Separation
- The width of a solute peak is important in
determining how well one solute is separated from
another - One measure of this is the width of the peak at
half-height (w½ ) or at its baseline (wb)
27
Introduction to Analytical Separations
- Chromatography
- 5.) Efficiency of Separation
- The separation of two solutes in chromatography
depends both on the width of the peaks and their
degree of retention
28
Introduction to Analytical Separations
- Chromatography
- 5.) Efficiency of Separation
- Resolution (Rs) is defined as
- Or
where tr2,tr1 retention times of solutes 1 and
2 (tr2 gt tr1) wb2,wb1 baseline widths of
solutes 1 and 2
where N number of theoretical plates g
t2/t1 (ggt1)
29
Introduction to Analytical Separations
- Chromatography
- 6.) Measure of Column Efficiency
- Number of Theoretical Plates (N)
- Similar to number of extractions performed in an
extraction separation - As N increase (number of separating steps) ?
greater the separation between two compounds
where wb baseline width of peak (in time
units) w1/2half-height peak width
30
Introduction to Analytical Separations
- Chromatography
- 6.) Measure of Column Efficiency
- Height Equivalent of a Theoretical Plate (H or
HETP) - The distance along the column that corresponds to
one theoretical separation step or plate (N)
where L length of column N number of
theoretical plates
H
31
Introduction to Analytical Separations
- Chromatography
- 6.) Measure of Column Efficiency
- H is affected by
- Flow-rate of mobile phase
- Size of support decrease size? decrease H
- Diffusion of solute increase diffusion ?
decrease H - Strength of retention
- Others
Improved resolution by increasing column length
32
Introduction to Analytical Separations
- Chromatography
- 6.) Measure of Column Efficiency
- Example
Two compounds with partition coefficients of 15
and 18 are to be separated on a column with Vm/Vs
3.0 and tm 1.0 min. Calculate the number of
theoretical plates needed to produce a resolution
of 1.5
33
Introduction to Analytical Separations
- Chromatography
- 7.) Why Bands Spread?
- Remember Efficiency is dependent on peak width
- A band of solute spreads as it travels through
the column - described by a standard deviation (s)
- Factors include
- Sample injection
- Longitudinal diffusion
34
Introduction to Analytical Separations
- Chromatography
- 7.) Why Bands Spread?
- Sample injection sample is injected on the
column width a finite width, which contributes to
the overall broadening - Similar broadening may occur in the detector
- Longitudinal diffusion band slowly broadens
- as molecules diffuse from high concentration
- in band to regions of lower concentration
35
Introduction to Analytical Separations
- Chromatography
- 7.) Why Bands Spread?
- Finite Equilibration Time Between Phases a
finite time is required to equilibrate between
stationary and mobile phase at each plate - Some solute is stuck in stationary phase as
remainder moves forward in mobile phase - Results in band broadening
Distribution of solute between mobile and
stationary phase
Solute in mobile phase moves down column ?
broader peaks
36
Introduction to Analytical Separations
- Chromatography
- 7.) Why Bands Spread?
- Multiple Flow Paths As solute molecules travel
through the column, some arrive at the end sooner
then others simply due to the different path
traveled around the support particles in the
column that result in different travel distances.
Molecules exit the column at different times due
to different path lengths
Molecules enter the column at the same time
37
Introduction to Analytical Separations
- Chromatography
- 8.) Description of Band Spread
- Plate height (H) is proportional to band width
- The smaller the plate height, the narrower the
band
Van Deemter equation
equilibration time
Multiple paths
Longitudinal diffusion
where mx linear flow rate A,B,C constants
for a given column and stationary
phase
38
Introduction to Analytical Separations
- Chromatography
- 9.) Types of Liquid Chromatography
- Adsorption Chromatography
- Solutes are separated based on their different
abilities to adsorb to the supports surface
39
Introduction to Analytical Separations
- Chromatography
- 9.) Types of Liquid Chromatography
- Partition Chromatography
- Solutes are separated based on their different
abilities to partition between the stationary
phase and mobile phase. - Uses a solid support coated or chemically
derivatized with a polar or non-polar layer
40
Introduction to Analytical Separations
- Chromatography
- 9.) Types of Liquid Chromatography
- Ion-Exchange Chromatography
- Used to separate ions based on their different
abilities to interact with the fixed exchange
sites. - Uses a solid support containing fixed charges
(exchange sites) on its surface
41
Introduction to Analytical Separations
- Chromatography
- 9.) Types of Liquid Chromatography
- Size Exclusion Chromatography
- Separates large and small solute based on their
different abilities to enter the pores of the
support - Uses a porous support that does not adsorb
solutes
42
Introduction to Analytical Separations
- Chromatography
- 9.) Types of Liquid Chromatography
- Affinity Chromatography
- Separates molecules based on their different
abilities to bind to the affinity ligand - Uses a support that contains an immobilized
biological molecule (affinity ligand)
43
Introduction to Analytical Separations
- Chromatography
- 9.) Types of Liquid Chromatography
- Packed and Open Tubular Columns
- Open tubular columns