Bacteriorhodopsin - PowerPoint PPT Presentation
Title:
Bacteriorhodopsin
Description:
Bacteriorhodopsin – PowerPoint PPT presentation
Number of Views:1565
Avg rating:3.0/5.0
Title: Bacteriorhodopsin
1
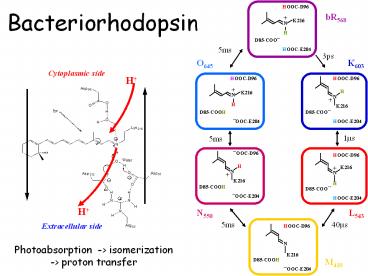
Bacteriorhodopsin
HOOC-D96
bR568
K216
N
H
D85-COO
5ms
HOOC-E204
3ps
K603
O645
Cytoplasmic side
HOOC-D96
HOOC-D96
H
K216
N
N
H
K216
D85-COO
D85-COOH
HOOC-E204
OOC-E204
1ms
5ms
OOC-D96
HOOC-D96
H
K216
N
N
K216
H
D85-COOH
D85-COO
OOC-E204
HOOC-E204
L543
N550
40ms
5ms
Extracellular side
HOOC-D96
N
Photoabsorption -gt isomerization -gt proton
transfer
K216
D85-COOH
M410
OOC-E204
2
Chromophore-binding Pocket
counterions
Aromatic amino acids
3
Proton accessibility!
cytoplasmic
H
photo-isomerization
all-trans
500 fs
extracellular
13-cis
4
All structural details in the retinal chromophore
are functionally important
Protonated Schiff base
Methyl groups
conjugated backbone
PA EAH EA
b-ionone ring
5
Delocalization of positive charge
6
Isomerization barriers in retinal
Low barriers against double bond isomerization
Ground state isomerization
7
A twisted chromophore in bR?
- A twisted chromophore is also experimentally
reported. - X-ray structures of bR report the twisted form of
chromophore - The twist is found around the terminal double
bonds - It may influence pKa of the chromophore
8
Photocycle of bR
Photo-induced
5 ms
3 ps
1 ms
5 ms
40 ms
5 ms
All intermediates are trapped in low temperature
and have been characterized by vibrational and
absorption spectroscopy.
9
Ultrafast spectroscopy
1 fs 3 x 10-4 mm 1 ps 3 x 10-1 mm
10
Ultrafast spectroscopy of bR
11
Ultrafast spectroscopy of bR
Femtosecond time resolution
H----I460----J625
Kobayashi et al., Nature, Nov 2001
12
Calculation of the Excited state Dynamics of
Photoactive Molecules by ab initio Techniques
Ab initio (First-Principles) dynamics of ethylene
in vacuum
Todd Martinez, Chemistry, UIUC
LUMO
50 fs
110 fs
0 fs
HOMO
But what happens in the protein?
13
QM/MM calculations
Lys216-RET
O
MM
N
H
N
H
MM
QM
QM
H
H
N
H
H
QM
dummy atom
Asp85, 212
O
O
H
O
N
QM
MM
14
Coupling of electronic excitation and
conformational change in bR
13
7
9
11
15
15
Hydrogen bond network in the retinal binding
T89
K216
N
O
O
Y185
H
H
H
O
O
O
H
H
D85
O
D212
W402
500 fs
O
H
O
H
W401
H
H
O
O
W406
H
H
Y57
H
N
R82
16
Water movement after the photoisomerization
Structure of the first intermediate (1QKO.pdb)
Structure of the ground state (1C3W.pdb)
C13C14 cis
13-cis
3 ps
One water molecule is dislocated, but where is
it?
17
Early intermediates of bRs photocycle
3ps
80ps
BR
K77K
KL135K
N-H 270o (T89)
N-H 90o (D212)
FTIR spec. (77K)
X-ray (110K)
(e.g., T89-D85 stronger)
(W402 is dislocated, D85 is rotated)
DEEX (kcal/mol)
-4.0 (-1.8)
-2.9 (-1.8)
18
Mechanism of Switching
T89
T89
O
O
N
N
H
H
H
H
O
O
O
-
-
O
O
H
H
O
C
C
-
-
D85
D212
D85
D212
H
H
C
C
O
O
O
O
H
H
BR
K
O
O
H
H
T89
T89
O
O
..
H
N
N
H
H
H
O
O
-
-
O
O
C
C
-
-
D85
D212
D85
D212
C
C
O
O
O
O
KL
L
H
H
H
H
O
O
19
Role of water in proton transfer
QM/MM calculation
proton transfer
neutral
zwitter -ionic
energy (kcal/mol)
Rearrangement of the hydrogen-bond network can
induce the proton transfer.
isomerization
20
THE PURPLE MEMBRANE
21
The Purple membrane of Halobacterium salinarum
22
Archaeal Membranes
- Branched (less vulnerable to oxidation)
- Etheric bridge, not esteric (less sensitive to
hydrolysis) - Inverted glycerol stereochemistry
Higher resistance to harsh conditions of their
habitat pH, heat, high salt and sulfur,
23
MODELING OF THE INTEGRAL PURPLE MEMBRANE
Bacteriorhodopsin trimer
Internal water molecules
Retinal chromophores
Squalene molecules
Intra-trimer lipids
Bulk water
Inter-trimer lipids
24
Charge distribution at different faces of the
purple membrane
Extracellular
Cytoplasmic
Basic Acidic Polar Lipids
25
Kinetics of the photocycle is dependent on the
lipid composition of the membrane
We have 10 molecules of lipid per bR monomer
PGP and squalene are necessary for the recovery
of normal kinetics of the photocycle after
detergent treatment of PM.
26
Helix dislocation at late stages of the photocycle
Possible involvement of lipid-protein interaction
in the photocycle