EMR info PowerPoint PPT Presentation
1 / 56
Title: EMR info
1
EMR info
0
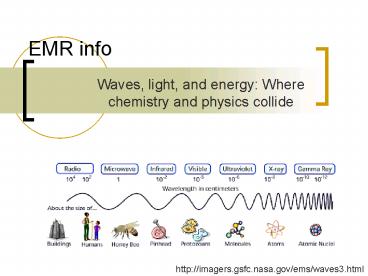
Waves, light, and energy Where chemistry and
physics collide
http//imagers.gsfc.nasa.gov/ems/waves3.html
2
0
First things first Waves
a and b represent wavelength (?)- the distance of
a wave from crest to successive crest measured
in meters
http//www.lbl.gov/MicroWorlds/ALSTool/EMSpec/EMSp
ec.html
3
Waves amplitude
0
- The height of a wave from crest to midline or
trough to midline measured in meters
4
Terms you need to know
0
- Wavelength (?)
- Amplitude
- Frequency (v I know some of you have used f,
move on and get with chemistry!) - the number of cycles per second
- measured in cycles per second (s-1) or Hz (Hertz)
- Propagation of electromagnetic waves
5
0
http//www.lbl.gov/MicroWorlds/ALSTool/EMSpec/EMSp
ec2.html
6
0
http//micro.magnet.fsu.edu/primer/lightandcolor/i
mages/electromagneticfigure1.jpg
7
0
http//lepus.physics.ualr.edu/tahall/EXAM2/emspec
.jpg
8
0
http//www.arpansa.gov.au/images/emsline2.gif
9
Visible Light
0
- color wavelength(Å) f(1014 Hz) Energy
(10-19 J) - Violet 4000---4600 7.5--6.5 5.0--4.3
- Indigo 4600---4750 6.5--6.3 4.3--4.2
- Blue 4750---4900 6.3--6.1 4.2--4.1
- Green 4900---5650 6.1--5.3 4.1--3.5
- Yellow 5650---5750 5.3--5.2 3.5--3.45
- Orange 5750---6000 5.2--5.0 3.45--3.3
- Red 6000---8000 5.0--3.7 3.3--2.5
10
Some equations you need to know
0
- ? c / v
- and
- E hv
- So.
- E hc / ?
- And
- ? h / mv
- When
- ? wavelength in m
- c speed of light, 3.00E8 m/s
- v frequency in Hz
- (cycles/sec or s-1 or 1/s)
- E energy in J
- h Planks constant, 6.626E-34 Js
Joule(seconds) - m mass of particle in kg
11
What the h? Plancks Constant
0
- When metals are heated, they glow
- 1800s- physicists were trying to determine the
relationship between the color (wavelength) and
intensity of the glow - Max Planck (1900)- energy can be released or
absorbed only in little chunks (packets) of
energy of some minimal size
12
Max Planck and the h
0
- The chunks of energy were dubbed quantum
(fixed amount), which is the smallest amount
that can be emitted or absorbed as EMR. - Proposed E hv
- The energy (E) of a single quantum is equal to
its frequency (v) times a constant
13
Planck and the Nobel (Physics)
0
- Planck determined that h 6.626E-34 J-s
- Energy is always released in multiples of hv
(1hv, 2hv, 3hv, etc) - h is so small that we cannot see the effects of
this in our daily lives - Analogous to
- Planck won the 1918 Nobel Prize in physics for
his work
14
Einstein Bohr Perfect Together
0
Einstein (or Mr., Read, you decide), left Bohr,
above
15
EinsteinThe Photoelectric Effect
0
- Einstein discovered that one could cause
electrons to be ejected from the surface of a
metal if the energy of the light wave was strong
enough - He treated the light needed to do this as a piece
of matter- a photon, if you will
- This ejection of e- is the photoelectric effect
16
The Photoelectric Effect
0
- Only light of a certain energy could knock off an
electron from the metal - Intense light of a weaker wavelength would not
work, but even a low intensity of the correct
wavelength would work - (the energy of the light is transferred to the
kinetic energy of the electron) - Hmmm light acting as a particle and as a wave..
17
The photoelectric effect
0
- http//www.lewport.wnyric.org/mgagnon/Photoelectri
c_Effect/photoelectriceffect1.htm - http//www.xmission.com/locutus/applets/Photoelec
tric.html
18
Getting to Bohr.
0
- Light of a given wavelength is monochromatic (one
color) - Most common EMR sources are polychromatic, but we
see only one color
- These can be reduced to a spectrum when the
different wavelengths are separated out
19
Spectral Emissions
0
- Continuous spectrum shows all colors of the
rainbow
20
0
- Bright line spectrum only certain wavelengths
are visible (the rest do not appear at all) - Different elements have different bright line
spectrum when they are heated - Na is yellow
- Ne is orange-red
21
Line spectrum
0
- Ne
- I2
22
0
http//www.cartage.org.lb/en/themes/Sciences/Astro
nomy/Modenastronomy/Interactionoflight/AtomicAbsor
ption/AtomicAbsorption.htm
23
Hydrogen Spectra
0
Emission Spectra
Absorption Spectra
http//www.mhhe.com/physsci/astronomy/applets/Bohr
/content_files/section1.html
24
0
http//www.cartage.org.lb/en/themes/Sciences/Astro
nomy/Modenastronomy/Interactionoflight/AtomicAbsor
ption/AtomicAbsorption.htm
25
0
26
0
- Line spectra formation- go to..
- http//www.mhhe.com/physsci/chemistry/essentialch
emistry/flash/linesp16.swf
27
Bohr Model and Spectral Emissions
0
- Bohr proposed that the emission of light energy
from an (electrically or thermally) excited atom
corresponds to the orbit of the electron around
the nucleus of the atom - That energy can only be achieved by being a
specific distance from the nucleus
28
What youve seen so far.
0
Model of a Nitrogen (z7) atom
29
Bohr Model and moving electrons
0
- http//www.colorado.edu/physics/2000/quantumzone/b
ohr.html
30
Energy levels- Bohr Model
0
- Electrons travel within set energy levels that
have a particular energy associated with each
level - After all, the e-s are moving around the nucleus
- think KE here
- Each shell has a number
- Closest to the nucleus is n1
- For each successive level add 1 to n
- n2, n3, ect.
31
Energy increases as the distance from the nucleus
increases
0
32
Bohr Model and moving electrons
0
- http//www.colorado.edu/physics/2000/quantumzone/b
ohr.html
33
Electron config in energy level
0
34
SO
0
- We know that the e--s are free to move around
the nucleus - They also can move from one energy level to the
next (and fall) back when energy is added - Move from ground state (home level) to a higher
level (the excited state) - Returning back to the ground state releases energy
35
0
- This emission is how we see colors
- the wavelengths of EMR released from an atom when
it has been excited by - Heat energy
- Electrical energy
- Chemical energy
- Think glowing red hot metal, or fireworks
36
Alsolife after Einstein and Bohr
0
- We know that electrons have characteristics of
both light (waves) and matter, so we say that
they have a dual nature
37
De Broglie
0
- De Broglie proposed that an electron moving about
the nucleus had a wave-like behavior, so that
they it has a particular wavelength associated
with it. This wavelength depends upon the mass
and velocity of the electron. - ? h / mv
- mv the momentum of the particle
38
0
- This matter-wave idea applies to all matter, not
just to electrons - However, the mass is so large, and the wavelength
so small, that we cannot see it in macroscale
objects - This matter-wave theory led to applications like
the electron microscope
39
De Broglie wavelength
0
40
HeisenbergThe Uncertainty Principle
0
- We cant determine information about small scale
objects the same way we can for large scale
objects - Case in point a ball rolling down a ramp- we can
get position, direction, and speed at the same
time - We cant for electrons
- Hence, the uncertainty principle
41
Heisenberg, contd
0
- It is inherently impossible for us to
simultaneously know both the exact momentum and
exact location of an electron - This is because anything we do to determine the
location or momentum of the electron moves it
from its original path and location this cant
be reduced past a certain minimal level - We can know only momentum or location- not both
- We can talk probability of the location/ momentum
of an electron
42
Which brings us to this question
0
- What the heck does all of this have to do with
electron configuration and how matter behaves? - On to electron configuration, courtesy of
Schrödinger and company (enter math that well
skip) - Quantum theory
43
EMR and the atom Part Deux
0
Electron Configurations
http//imagers.gsfc.nasa.gov/ems/waves3.html
44
What youve seen so far.
0
Model of a Nitrogen (z7) atom
45
Which is really not true- why?
- Because orbitals- the electron cloud are
- 3-D
- are not round in all most cases
- e- spread out as much as possible.
46
(No Transcript)
47
The s orbital
http//www.sfu.ca/nbranda/28xweb/images/s_orbital
.gif
48
p orbitals
49
d orbitals
50
d orbitals
51
f orbitals
52
General tutorials for electron configuration stuff
- some slides in this PowerPoint are from this site
already - http//www.wwnorton.com/chemistry/overview/ch3.htm
- See key equations and concepts (select from menu
on the left), as well as the looking through the
overview where to the tutorials are listed (links
for just those are on the left, too)
53
(No Transcript)
54
(No Transcript)
55
Quantum numbers
56
(No Transcript)