Lecture 19 PowerPoint PPT Presentation
1 / 13
Title: Lecture 19
1
Molecular Geometry
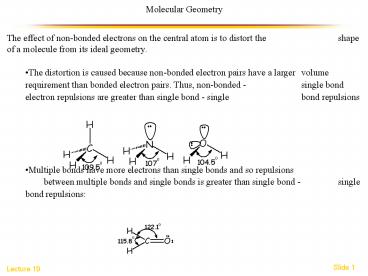
- The effect of non-bonded electrons on the central
atom is to distort the shape of a molecule from
its ideal geometry. - The distortion is caused because non-bonded
electron pairs have a larger volume requirement
than bonded electron pairs. Thus, non-bonded
- single bond electron repulsions are greater
than single bond - single bond repulsions - Multiple bonds have more electrons than single
bonds and so repulsions between multiple bonds
and single bonds is greater than single bond -
single bond repulsions
2
Molecular Geometry
- Trigonal bypyramidal molecules with non-bonded
electron pairs - There are two kinds of sites in these molecules
for bonded atoms and non- bonded electron pairs - Groups in the trigonal plane have two 120o
neighbors and two 90o neighbors. - Groups in the axial positions have three 90o
nearest neighbors - Because non-bonded electron - bonded electron
repusions are greater than bonded electron -
bonded electron repusions, non-bonded electrons
will find themselves in the equitorial trigonal
plane of the trigonal bipyramid
3
Molecular Geometry
Molecules with more than one central atom are
analyzed one atom at a time
4
Molecular Geometry
- Electric Dipole moments whenever there is a
charge separation in a molecule the molecule has
an electric dipole moment. Molecules without any
net charge separation within the molecule is
nonpolar. - All heteronuclear diatomic molecules are polar
because of the electronegativity difference
between the bonded atoms.
5
Molecular Geometry
Bond lengths, electronegativity differences and
dipole moments of the hydrogen halides
6
Molecular Geometry
- Dipole moments for polyatomic molecules depend on
the magnitude and direction of the individual
bond dipole moments in a molecule. - Dipole moments are vector quantities
- Both the magnitudes and directions of the
individual moments must be summed.
CO2 is nonpolar because the two bond moments are
of equal magnitude but point in exactly the
opposite directions and cancel
Bond moment
Bond moment
H2O is polar because the two bond moments do not
cancel but co-add to give a net molecular dipole
moment
Total molecule moment
NH3 is polar
BF3 is nonpolar
CH4 is nonpolar
SF4 is polar
7
Molecular Geometry
Dipole moments of some molecules
8
Chemical Bonding Theory
- Valence bond theory is one of two methods of
viewing how electrons are shared in covalent
bonding. - The quantum mechanical approach to valence bond
theory is that the wave function associated with
the shared electrons is made up from the atomic
orbitals on the two bonded atoms so that their
identity is retained. - Electrons are localized in the region where the
bond forms - The atomic orbitals overlap so as to give a
maximum in their overlap and put as much
electron density as possible between the bonded
atoms. - This is consistent with the Lewis model which
places the bonding electrons between the boned
atoms
9
Chemical Bonding Theory
- Valence bond theory
- H2 is a simple example each H atom has a 1s
electron - The two electrons are shared equally in each
atoms 1s orbital
The next slide shows how the potential energy of
the two atoms changes as they are brought
closer together from infinite separation The
minimum potential energy occurs when the nuclei
are 74 pm apart
10
Chemical Bonding Theory
11
Chemical Bonding Theory
- For the heternuclear diatomic HF, the bond
results from overlap of the 1s orbital on H and
the half-filled p orbital on - HF
In terms of the valence bond theory, the bond is
formed by pairing the 1s electon from H with the
2p electron from F to form the electron pair bond.
12
Chemical Bonding Theory
- For H2O, one valence bond picture is that the 1s
electrons on each H atom overlaps with two
half-filled p orbitals on O to form two electron
pair bonds.
For clarity, only the two 2p orbitals on O
involved in bonding are shown. There is also a 2s
and a third 2p valence orbital on O, each with a
pair of electrons. Note this picture predicts a
90o H-O-H bond angle in water. The actual bond
angle is 104.5o and the deviation could come from
the d charges on H due to the electronegativity
difference between H and O.
13
Chemical Bonding Theory
- Valence bond theory
- For NH3 a similar picture gives three electron
pair bonds from overlap of the three 1s
electrons on each H atom and the three 2p
orbitals on N each with one unpaired electron.
- N 1s22s22px12py12pz1
- This picture predicts
- The H-N-H bond angle of 90o giving a trigonal
pramidal structure. - The non-bonded valence electron pair is in a 2s
orbital