Hydroamination PowerPoint PPT Presentation
1 / 35
Title: Hydroamination
1
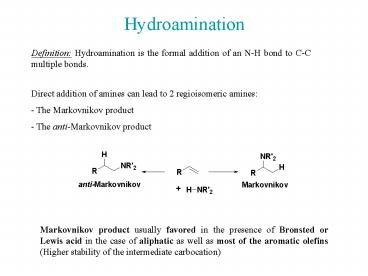
Hydroamination
Definition Hydroamination is the formal addition
of an N-H bond to C-C multiple bonds.
Direct addition of amines can lead to 2
regioisomeric amines - The Markovnikov product -
The anti-Markovnikov product
Markovnikov product usually favored in the
presence of Bronsted or Lewis acid in the case of
aliphatic as well as most of the aromatic olefins
(Higher stability of the intermediate
carbocation)
2
Hydroamination
But several thermodynamic and kinetic aspects
make the direct nucleophilic addition of amines
across C-C multiple bonds difficult.
- The nucleophilic attack of the amine nitrogen,
bearing the lone pair, on the electron rich
non-activated multiple bonds leads to
electrostatic repulsion. - The high energy difference between p (CC) and s
(N-H) orbitals forbids a thermal
22cycloaddition of the N-H bond and the
alkene. - The hydroamination reaction is only slightly
exothermic or even thermoneutral. - Because of the highly negative reaction entropy,
the reaction is not favored at high temperatures.
3
Hydroamination
Thus Direct nucleophilic addition of amines
proceeds easily only to electron-deficient
(activated) p-systems with functional groups,
such as keto, ester, nirile, sulfoxide or nitro
leading to the anti-Markovnikov products
4
Hydroamination - Mechanisms
How can the hydroamination reaction be promoted?
- C-C multiple bonds can be activated towards
hydroamination by late transition metals. - The amine can be activated by oxidative addition
to a late transition metal, which allows
insertion of the alkene into the M-N or M-H bond,
thereby promoting the hydroamination
catalytically. - Strong bases or strongly electropositive metals
like alkali, alkaline earth, or the lanthanide
group elements, can deprotonate amines to give
more nucleophilic amides, which can undergo
addition to certain olefins. - The amine can be activated by being converted
into the coordinated imide MNR using early
transition metal complexes or Actinides and the
reaction of C-C multiple bonds with the M-N bond
can then occur.
5
Hydroamination - Mechanisms
I) Ammination via activation of olefins
The nucleophilic attack of amines on the
unsaturated C-C bond is facilitated by
coordination of the olefin (or alkyne) to an
electrophilic transition metal center Pd(II),
Pt(II), Hg(II), Mo(II), Fe(II).
- b-hydride elimination
Oxidative amination product
- Protonolysis
Hydroamination product
6
Ammination via activation of olefins
A) Stoichiometric use of Transition Metals
To overcome the problem that amines are strong
ligands for electrophilic TM centers (they can
rather displace than attack the coordinated
olefin), aminations have been performed in the
presence of stoichiometric amounts of TM. The
amine-olefin complexes can also sometimes be
isolated.
Whereas these complexes are stable towards
isolation, the alkene palladium(II) complexes are
much more reactive and their stability is
dependent on the steric nature of the olefins.
7
Ammination via activation of olefins
Two different routes for the Nucleophilic Attack
on Coordinated Olefins
Most amination reactions
Route A Metal trans to Nucleophile
8
Ammination via activation of olefins
The rate of addition of amine is also dependent
on the steric nature of the olefin
Markovnikov addition the least hindered metal
alkyl complex is formed Except in few cases where
steric factors of the amine substituents dominate
the reaction.
9
Ammination via activation of olefins
B) Making the reaction catalytic
- Two Different Strategies
- The (2-aminoalkyl)metal complex must undergo
protonolysis to regenerate the catalytically
active transition-metal complex, or - After reductive elimination from the 2-aminoalkyl
complex the resulting transition metla complex
in a low oxidation state has to be reoxidized to
the active catalyst species typically 5-10 of
Pd(II) are used in the presence of benzoquinone,
CuCl2/O2 or more recently O2/DMSO as reoxidant
10
Ammination via activation of olefins
Palladium-catalysed Markovnikov hydroamination of
vinylarenes
-The reaction occurred with electron-rich
anilines in high yields. -The reaction with an
electron-poor anilines occurred in lower
yield. -Reactions with electron-poor vinylarenes,
such as trifluoromethylstyrene, occurred with the
fastest rates and in the highest yields.
11
Ammination via activation of olefins
Rhodium-catalysed anti-Markovnikov hydroamination
of vinylarenes
Reactions of the electron-neutral or the
electron-rich vinylarenes occurred with high
ratios
12
Hydroamination - Mechanisms
II) Ammination via activation of Amine
A) Catalytic hydroamination of olefins via
oxidative addition of the amine to a transition
metal
13
Hydroamination - Mechanisms
II) Ammination via activation of Amine
B) Based-catalysed amination of olefins via metal
amide species
14
Based-catalysed amination of olefins
- The nucleophilic addition of the metal amide to
the olefin is the rate determining steo (step
II) high activation energy due to the
unfavorable interaction between the nitrogen lone
pair and the p-system of the alkene. - The amination is strongly dependent on the pKa of
the amine used - great pKa high basicity or nucleophilicity of
a low temperature - Most favourable conditions for the
hydroamination - High concentrations of catalyst and olefin
- Low acidity or high pKa of the amine and high
nucleophilicity of the metal amide complex which
can be achieved by choosing suitable metal
precursors, solvents and certain additives.
15
Based-catalysed amination of olefins
The addition of TMEDA usually increases the rate
of reaction this is an example of
ligand-accelerated catalysis which has been
explained by the formation of a lithium complex.
16
Based-catalysed amination of olefins
17
Based-catalysed amination of olefins
1,3-Dienes
Nucleophilic addition of amines to 1,3-dienes
primarily leads to the 1,4-addition products. The
stereochemistry of the products varied from
predominantly E to nearly exclusively Z depending
on the structure of the amine used and the
solvent employed.
18
Based-catalysed amination of olefins
1,3-Dienes
19
Based-catalysed amination of olefins
Styrene
The addition of amine to styrene catalyzed by
lithium amide proceeds regioselectively in
anti-Markovnikov fashion to produce
1-amino-2-phenylethane.
20
Based-catalysed amination of olefins
Aryl olefins
21
Hydroamination - Mechanisms
II) Ammination via activation of Amine
C) Catalytic hydroamination of alkynes (or
alkenes) via metal imide species
This process is very often used with alkynes to
form enamines and imines but also with
aminoalkenes and amino alkynes to form 5-, 6- or
even 7-membered rings. Aminoalkenes
H2N(CH2)nCHCH2 (n4, 5) can be cyclized with
various titatium, zirconium, lanthanide (Ln) and
Actinide (Ac) complexes to yield pyrrolidines and
piperidines, respectively.
22
Hydroamination of alkynes (or alkenes) via
metal imide species
Intramolecular Aminoalkene and Aminoalkyne
HA/Cyclisation mediated by Organolanthanide
complexes
23
Organolanthanide-Catalysed Intramolecular
Hydroamination
24
Hydroamination of alkynes (or alkenes) via
metal imide species
Intramolecular HA/Cyclisation of Terminal
Disubstituted Aminoalkenes, Aminoalkynes,
Aminoallenes, and Aminodienes
25
Hydroamination of alkynes (or alkenes) via
metal imide species
Intermolecular HA of Alkynes with Bulky Amines
26
Intermolecular HA of Alkynes Allenes
Zirconium Complexes
In each case the favored hydroamination product
bears the smaller alkyne substituent located a to
the nitrogen atom.
27
Intermolecular HA of Alkynes Allenes
Titanium Complexes
28
Intermolecular HA of Alkynes Allenes
Titanium Complexes
29
Intramolecular HA of Alkynes Allenes
Titanium Complexes
30
Asymmetric Hydroamination of Alkenes
Rare earth metal catalysts for the
diastereoselective and enantioselective
hydroamination of aminoalkenes
31
Asymmetric Hydroamination of Alkenes
Rare earth metal catalysts for the
diastereoselective and enantioselective
hydroamination of aminoalkenes
32
Asymmetric Hydroamination of Alkenes
Plausible cyclisation transition states for the
preferred formation of trans-2,5-dimethyl-pyrrolid
ine
33
Enantioselective Hydroamination/Cyclisation
34
Enantioselective Hydroamination/Cyclisation
35
Asymmetric Hydroamination of Alkenes
Asymmetric Aza-Michael addition of Amines to
a,b-Unsaturated Amides
The role of the catalyst is limited to activate
the olefinic sbstrate through Lewis acid
coordination fro nucleophilic attack of the amine