Directional forces - PowerPoint PPT Presentation
1 / 16
Title:
Directional forces
Description:
van der Waals (Dispersion) Forces. Array of molecules, which have a temporary dipole ... This equation allows us to find the 'van der Waals radii' of atoms. ... – PowerPoint PPT presentation
Number of Views:110
Avg rating:3.0/5.0
Title: Directional forces
1
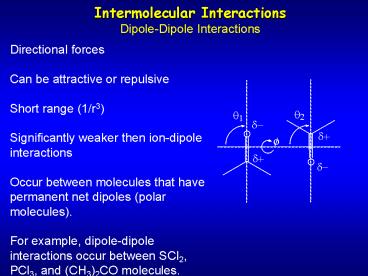
Intermolecular InteractionsDipole-Dipole
Interactions
Directional forces Can be attractive or
repulsive Short range (1/r3) Significantly
weaker then ion-dipole interactions Occur
between molecules that have permanent net dipoles
(polar molecules). For example, dipole-dipole
interactions occur between SCl2, PCl3, and
(CH3)2CO molecules.
f
2
Intermolecular InteractionsDipole-Dipole
Interactions
- Energy - (k . u1 . u2 / e . r 3) . ((2
cosq1cosq2 - sinq1sinq2) . cosf) - Maximum when dipoles have in-line
configuration, where q 0 - Simplifies to
- - 2k . u1 . u2 / (e . r 3)
3
Intermolecular InteractionsDipole-Dipole
Interactions
- Example Two acetone molecules in chloroform in
head to tail arrangement separated by 0.5 nm - -2k . u1 . u2 / (e . r 3)
- -2 . 9 . 109 . (2.88 . 3.34 .10-30 )2 / (4.8 .
(0.5.10-9)3) - -28.08 . 10-22 J
- -1.68 kJ/mol -0.4 kcal/mol
4
Intermolecular Interactionsp-p interactions
5
Intermolecular Interactionsp-p interactions
Distribution of electron density in benzene
molecule
6
Intermolecular Interactionsp-p interactions
7
Intermolecular Interactionsp-p Stacking
H
H
H
-
-
-
6d
H
H
H
H
H
Edge-to-face
8
Intermolecular Interactionsp-p Stacking
Crystal structure of benzene
Herringbone packing of anthracene molecules
9
Intermolecular Interactionsp-p interactions
Offset, face-to-face
Face-to-face, not favorable
10
Intermolecular InteractionsCation-p Interactions
H
H
-
H
H
6d
H
H
11
Intermolecular InteractionsCation-p interactions
and p-p stacking
Binding of anti-Alzheimers drug Aricept to
active site of acetylcholinesterase from Torpedo
californica
12
Intermolecular Interactionsvan der Waals
(Dispersion) Forces
- Bond energy is very weak (0.1-1 kcal/mol)
- Exists between almost all atoms and molecules
- Arise from atomic or molecular dipoles
- Interactions between the fluctuating induced
dipoles (due to instantaneous and short-lived
vibrational distortions)
13
Intermolecular Interactionsvan der Waals
(Dispersion) Forces
This transient polarization will continue to
fluctuate because of the electron movement, but
the induced polarities are synchronized so that
the attraction from the polarity is maintained as
long as the molecules are close together.
14
Intermolecular Interactionsvan der Waals Forces
Often described as dispersion forces, i.e.
instantaneous dipole interactions. More general
definition includes ion-induced dipole, and
dipole-induced dipole interactions. Describes the
ubiquitous weak attraction between all atoms. E
-A/r6 Lennard-Jones potential combines vdw forces
with the hard sphere repulsion (B/r12) between
atoms at very close distance. E B/r12 -
A/r6 This equation allows us to find the van der
Waals radii of atoms. This is the size of an
atom or the preferred distance atoms will pack to
if there are no other significant interactions.
15
Intermolecular Interactionsvan der Waals
(Dispersion) Forces
Strength of interaction is essentially a function
of the surface area of contact and the
polarizability of electron shells. The larger the
surface area the stronger the interaction will
be. Regardless of other interactions found
within a complex there will always be a
contribution from vdw. This is what drives
molecules to eliminate spaces or vacuums and
makes it difficult to engineer porous or hollow
structures and gives rise to the phrase Nature
abhors a vacuum.
16
Intermolecular Interactionsvan der Waals Forces
Induced dipole-induced dipole
Three main types of VDW interactions