ZiyyaraEdutech PowerPoint PPT Presentation
Title: ZiyyaraEdutech
1
Modern Periodic Classification of Elements
Written by Ziyyara 29-04-2020
Enroll Now for Online Tuition
Modern Periodic Classification of Elements
As we all know that the whole universe
constitutes chemistry in it. In the form of the
smallest unit that is atom, and after it comes to
molecules and modern periodic classification of
elements. The entire things which are there in
the universe are made up of elements. So to
understand the chemistry around us first there
is a need to understand its basic unit that is
element. Anything can be understood well if
there is a proper system. And to understand the
Classification of Elements there physical and
chemical properties there is a need of
classification.
2
So, to understand the properties of elements we
have classified modern periodic classification
of elements. Various scientists have done their
contribution in this regard with the elements of
their time. As there is on-going discovery of
elements taking place till now as well. Thats
why modern periodic classification of elements
has an important stand in chemistry. Now let us
begin the journey with the ancient to recent one
classification. Dobereiner's triads in Periodic
Classification of Elements In Dobereiner's
triads- In 1817, a chemist decided to manage an
element in a table of a triad in which he
arranged elements in the order of atomic masses
that the atomic mass of the middle element is the
average of the first and third element in the
triad.
Group A element Atomic masses
Nitrogen 14.0
Phosphorus 31.0
Arsenic 74.09
Group B element Atomic masses
Calcium 40.1
Strontium 87.6
Barium 137.3
3
- (In first triad phosphorus mass is average of
nitrogen and arsenic mass in second strontium
has atomic mass which is a average of calcium and
barium) - Limitation of Dobereiner Triads in Periodic
Classification of Elements Lesson - The limitations of Dobereiners triads are as
follows- - The discovery of new element has ruled out the
triads law - Newly discovered elements are not satisfying the
rule. - Only 5 Dobereiners triads were identified.
- Several of the known elements were not fit into
the triad. - After knowing these limitations, the new
classification development had occurred. - Newlands Octaves law in Periodic Classification
of Elements 10th Class - This idea of the periodic table consists of the
idea of the law of octaves of the music of West
like (do, re, mi, Fa, so, la, ti. Comparatively
sa, re, Ga, ma, pa, Dha, ni (in Indian style
way), according to his idea like the musical
notes repeat itself in the same way in
classification every eighth element in the order
of their increasing atomic masses have its
property repetition with the first element. - Indian and Western Musical Octaves for Elements
Table
SA R e G a Ma P a D h a N i
Do R e M i Fa S o L a T i
4
H L e B i B C N O
F N a M g Al S i P S
Cl K C a Cr T i M n F e
Co Ni C u Z n Y I n A s S e
Br R b S r Ce La Z r
- Limitations of Octaves Law of Newland While
Studying Periodic Classification of Elements - After calcium, there is no element that seems to
have the same property as per the conditions of
the law. - He imagined that only 56 elements are present in
the whole world which had been included in the
table as well. - Placement of some elements like Nickel and cobalt
in the same slot where both are different in
chemical properties. It created a fuss here to
understand the table. - Discovered some noble gases in the environment
like helium, beryllium, etc. This also affected
the table because there is no place for place
them in the table.
5
Mendeleev Periodic Table One of the important
names when it comes to modern periodic
classification of elements, Mendeleev
contribution is enormous in terms of
classification. In 1869, when octave law was
rejected. Then one of the great scientists
Mendeleev, his periodic table was came into
effect. In his periodic table atoms were
arranged based on their fundamental property,
atomic mass, and chemical property. In his time
of formulation only 63 elements were known, so
he made his periodic table with those 63
elements. Mendeleevs table was divided into
horizontal rows and vertical columns. For his
arrangement of elements in the periodic table he
made its formulation with oxygen and hydrogen
(oxides and hydrides) then he wrote all his
observation on the cards (formula with oxides,
hydrides and atomic mass) he grouped together the
element which had the similar properties he
found that element with similar properties were
occupying same vertical columns and that similar
trend were observed in horizontal rows too In
his observations he formulated the periodic law
which states that The property of elements is a
periodic function of their atomic masses He
named the vertical column as groups and
horizontal rows as period.
6
- Standards of Mendeleev Periodic Table of Elements
- Some gaps were left in the table for the element
which were not discovered at that time so he
said that the element which will be discovered
later will occupy this position in the table
without disturbing the existing sequence. - The table was found in the sequence of atomic
masses as we can see in the table. - But some of the elements were placed before any
other slightly light element of the table. For
example cobalt (along with an atomic mass of
58.9) was placed slightly higher than a nickel
(along with the atomic mass of 58.7) and even we
can see such anomaly in the periodic table
classification of elements. - Eka - word of Sanskrit was emphasized by the
Mendeleev to that undiscovered element in that
time. It means that the undiscovered element
would be mentioned in the hollow cards place or
the unfilled slots of the periodic table of
elements.
7
For example- gallium, scandium, and germanium
and other element were discovered later, they
would add in the classification of the periodic
table of the element in the Sanskrit style of
Eka-silicon, Eka-boron, Eka-aluminium and other
further discovered elements like this. Further
discovered element of aluminium would be
discovered mentioned as Eka-aluminium and later
discovered as the -gallium in the table of the
elements of the periodic table.
Property Eka- aluminium Galli um
Atomic mass 68 69.7
Formula of oxides E2O3 Ga2 O3
Formula of chlorides ECl3 GaCl 3
- Limitations of Mendeleev Modern Periodic
Classification of Elements Table - He was not able to assign proper position for
hydrogen - The gradation in atomic masses was not regular
there was no number prediction of how many
elements will occupy the empty spaces. - After the discovery of isotopes there was
violation in Mendeleevs periodic law
Modern Periodic Table of Elements To make the
classification system easier Henry Mosley gave
the concept of ATOMIC NUMBER Hence, atomic
number became the fundamental property of the
element in the modern periodic table.
8
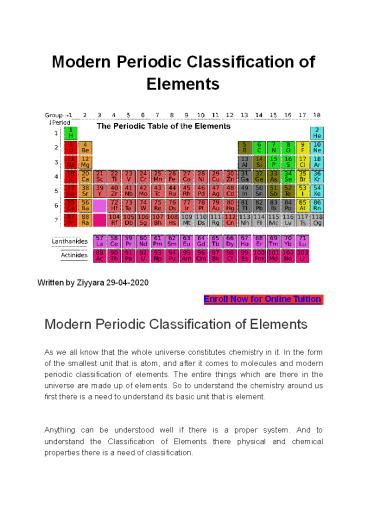
- Fundamental things to be noted for the Modern
Mendeleev Periodic Table of Elements - Horizontal columns as periods total 7 in the
list of the table in which five are at the top
and two lanthanides and actinides at the bottom. - Vertical columns are known as groups they are
total 18 in number. - Group 18 contains noble gases.
- Each group has the same valency or valence
electron in the outermost shells.
Till now we have discovered the 118 elements in
modern periodic table in 2016 oganesson was
discovered and added in the modern periodic
classification of elements table. Position of
Elements in the Modern Periodic Classification
Table There are 18 groups and 7 horizontal
columns in the modern periodic classification of
elements table.
9
- The element with the same number of valence
electrons is there in the same group and as we
descend to the group the number of shells goes on
increasing. - The elements with different numbers of electrons
but the same number of valence shells (that
means the number of electrons in the valence
shell is increasing but the number of shells
remains same) were occupied in the same group. - Trends in the Modern Periodic Classification of
Elements Table - As we move top to bottom in a group the atomic
radius (the distance of outermost shell from the
nucleus) will go on increasing because the
numbers of shells are increasing, and as we move
left to right in a period the atomic size goes
on decreasing because nuclear force is
increasing. - At the left extreme there are metals (group12)
on the right side there are non-metals a zig zag
line separating the metalloid which has the
property of both metals and non- metals. - The effective nuclear charge is increasing from
left to right so the tendency to lose electron
is decreasing because they experience more
nuclear pull as we move down the group the
valence electron remain same but the number of
shells are increasing, so the effective nuclear
charge is decreasing and the tendency to lose
electrons is increasing so tendency to behave as
a metal is increasing along a group and
decreasing across a period.