Lecture 9b Nitrogen Cycle N2 gas into NO3 - PowerPoint PPT Presentation
1 / 36
Title:
Lecture 9b Nitrogen Cycle N2 gas into NO3
Description:
Problem is getting N2 into a form that plants can use. ... Gage and Margolin, 2000. Root hair curling around rhizobia. Rhizobia reproduce ... – PowerPoint PPT presentation
Number of Views:126
Avg rating:3.0/5.0
Title: Lecture 9b Nitrogen Cycle N2 gas into NO3
1
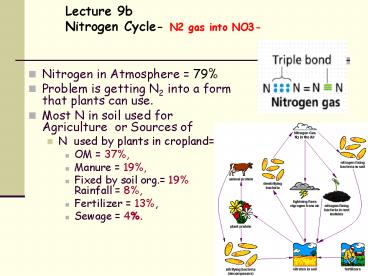
Lecture 9bNitrogen Cycle- N2 gas into NO3-
- Nitrogen in Atmosphere 79
- Problem is getting N2 into a form that plants can
use. - Most N in soil used for Agriculture or Sources
of - N used by plants in cropland
- OM 37,
- Manure 19,
- Fixed by soil org. 19 Rainfall
8, - Fertilizer 13,
- Sewage 4.
2
Nitrogen Fixation- Conversion of N2 into NH3 or
R-NH2
- . Non-Biological Fixation
- -Air Pollution -The main oxides of nitrogen
present in the atmosphere are nitric oxide (NO),
nitrogen dioxide (NO2) and nitrous oxide the
result of fuel combustion from motor vehicle
exhaust and stationary fuel combustion sources
like electric utilities and industrial
boilers--oxides of nitrogen may remain in the
atmosphere for several days and during this time
chemical processes may generate nitric acid, and
nitrates and nitrites as particles. - - Rainfall additions from electrical discharge
(lightning) 2-5 lbs....../acre/year - N2 -----gt NO3-
3
N2
Denitrification
N fixation
immobilization
R-NH2
NO3-
Plants
mineralization
Nitrification
Ammonification
NH4
4
Nitrogen FixationConversion of N2 into NH3 or
R-NH2
- . Biological Fixation
- 1. Non-Symbiotic (independent organism) -
Azotobacter - aerobic Clostridium - anaerobic
about 5-50 lbs....../acre/year - 2. Symbiotic - mutually beneficial for host
organism and bacteria - complex plant -
bacteria interaction http//www.agron.iastate.edu/
loynachan/mov/
5
B. Symbiotic N- Fixation
- Bacteria Rhizobia
- Plant Legume - peas, clover, alfalfa, cowpeas,
peanuts, beans, soybeans - Alfalfa - 200 lbs....../acre/year
- Soybeans - 100 lbs......./acre/year
- Beans - 40 lbs...../acre/year
- Green manure is live plant material added to
soil to increase N content and SOM.
6
Symbiotic N Fixation
- Bacteria invades host plant root
- Response of host plant root is to grow a nodule
for the bacteria to live in. - Bacteria takes N2 from the air and converts it
into R-NH2 which resides in Bacteria in Nodule
and some is in the form of NH4 - Fate of N Fixed by Rhizobium
- 1) used by host plant,
- 2) leaks out of root to become available to
surrounding plants, - 3) as roots and nodules are sloughed-off
heterotrophic organisms immobilize the N and it
eventually becomes part of the SOM.
7
Infection and nodule formation
Rhizobium
Alfalfa root nodule
Dazzo Wopereis, 2000
Root hair curling around rhizobia
Rhizobia reproduce in infection threads
M. Barnett
Bacteroids filling a single cell
Dazzo Wopereis, 2000
Michael Russelle - USDA-ARS Plant Science
Research Unit
Gage and Margolin, 2000
Vance et al., 1980
8
Michael Russelle - USDA-ARS Plant Science
Research Unit
Nitrogen fixation is (usually) reduced by
external N
Fixed N
Total N in the plant
Soil N
N2
N2
9
Legumes buffer the N supply and fix what they
need from the air
Legume
Legume
Grass
Grass
Fixed N
Manure N
Soil N
Michael Russelle - USDA-ARS Plant Science
Research Unit
10
We need to fertilize non-legumes and can easily
guess wrong
Legume
Legume
Grass
Grass
Loss
Fixed N
Fert N
Manure N
Soil N
Michael Russelle - USDA-ARS Plant Science
Research Unit
11
2. Ammonification
- A. Ammonification in the soil is the conversion
of organic N (RNH2) into inorganic ammonia (NH3)
heterotrophic organ.
- R-NH2 ---gt NH3 H ----gt NH4
12
B. Fates of NH4
- 1) fixed by clay minerals,
- 2) lost by soil erosion,
- 3) used by plants (NH4),
- 4) volatilization
- NH4 ----gt NH3
High pH Soils gt 7.5
13
3. Nitrification
- 2 - step process
- 1. 2NH4 3O2 ---gt 2NO2- 4H 2H20 E
- Nitrosomonas
- 2. 2NO2- O2 --gt 2NO3- E
- Nitrobacter
- Process is acid causing due to release of 4 H
14
3. Fates of Nitrate
- Immobilization ---gt Plant uptake of NO3-
- NO3- is not held by soil particles and is
easily leached - when ppm NO3- is gt 10 ppm the
water is considered to be contaminated - Denitrification - stimulated by anaerobic
conditions.
15
Active nitrate remediation in ground water
Dr. Mike Russelle- USDA/ARS Plant Research Unit
ET
Irrigation
Public water well
Crop N uptake
Leaching
Ground water flow
16
Small plot work at Becker
Established alfalfa, orchardgrass, bromegrass,
and soybean irrigated with high nitrate water (25
to 50 mg N/L) by drip irrigation during the
growing season (2 to 5 cm water/week)
Michael Russelle - USDA-ARS Plant Science
Research Unit
17
4. Denitrification
- Involves conversion of NO3- to N2 gas
- C6H12O6 4NO3- --gt 6CO2 6H2O
2N2(gas) NO NO2 - Bacteria anaerobic
- Through nitrification and denitrification 10 - 20
of the applied N is lost.
- Nitrification inhibitors can be applied like
N-Serve. This chemical inhibits the growth of
nitrosomonas
and nitrobacter or slows conversion of NH4
conversion to NO3-
18
N2
Denitrification
N fixation
immobilization
R-NH2
NO3-
Plants
Nitrification
Ammonification
NH4
19
Duxbury, 1997, Wm. C. Brown Publishers
20
Nitrate in drinkingwater supplies
- Nitrate has been detected in surface- and
ground-water supplies in various parts of the
state. - Low levels of nitrate can be found in most of the
surface waters of the state. - In a recent statewide survey of water wells, a
small percentage contained excessive nitrate
concentrations.
21
- In cases where the concentration of
nitrate-nitrogen exceeds the maximum contaminant
level of 10 mg/L, as set forth by the U.S. EPA -
water suppliers are required to issue a nitrate
alert to users. - The health of infants, the elderly and others,
and certain livestock may be affected by the
ingestion of high levels of nitrate.
USGS, 1998
Risk of Groundwater Contamination by Nitrate
22
CN Ratios
- Bacteria require about 5 grams of carbon for
each gram of nitrogen assimilated or used CN in
a ratio of 51. - Decomposing microorganisms have first priority
for any mineralized N. - This use of N by decomposers results in
insufficient N for plants. - Eventually period of N starvation is over after
all the high CN material is decomposed.
23
CN Ratio of some organic materials
- domestic sewage -51
- Muni. sewage - 81
- legume hay -131
- Mun. Compost 28 1
- green grass - 351
- corn stover - 501
- Straw - 801
- Sawdust - 4001
Break even point for CN is 20 to 30 1.
24
Decomposition Rate as measured by CO2
100
Grain, or canning waste
Cumulative CO2 Evol. as of C added in
organic material.
Alfalfa
50
Corn Stalks
newspapers, sawdust,leaves
0
pine needles
0
20
Time ( weeks)
25
N-Cycle
- Plants need NO3-
- This can be supplied as NO3-, NH4, or organic N
(R-NH2), - The rate at which NO3- is available depends on
CN, temp, O2, water,
26
Ohio State University Extension Fact Sheet
27
- If you had to dispose of 10 tons of sawdust every
month from a local saw mill, what would be your
solution?
28
Soil Inoculants to increase N Fixation
- Inoculate soil or seeds with N-fixing bacteria
- Introduce bacteria, nematodes, or insects that
are predators of pest organisms - Add nitrification inhibitors to reduce bacteria
that convert ammonium to nitrate
29
Composting
- A biological process that breaks down organic
material (such as grass clippings and leaves)
into more stable molecules
30
Stages of Composing Process
- Mesophilic stage 1
- Brief
- Temperature rises to 40 degrees C
- Sugars and readily available microbial food
sources are rapidly metabolized
31
Stages of Composing Process
- Thermophilic stage
- 50 to 70 degrees C
- Easily decomposed compounds are used up and
humus-like compounds are formed - Frequent mixing essential to maintain oxygen
levels and assure even heating of all material- - If too hot may kill organisms in the pile
32
Stages of Composing Process
- Mesophilic (2nd)
- Curing stage
- Temperatures fall back to ambient
- Material recolonized by mesophilic organisms
33
Benefits to Composting
- Safe storage
- Easier handling
- Volume reduced 30 to 50
- Material more uniform
- Nitrogen competition avoidance
- No nitrate depression
- Nitrogen stabilization
- N in organic form
34
Benefits to Composting
- Partial sterilization
- thermophilic stage kills most weed seeds and
pathogenic organisms in - Detoxification
- Most organic compounds are destroyed
- Disease suppression
- Compost suppresses soil borne diseases by
encouraging microbial antagonisms
35
(No Transcript)
36
The
End