Material Balances on Reactive Processes - PowerPoint PPT Presentation
1 / 9
Title:
Material Balances on Reactive Processes
Description:
What does a reaction do to the general balance equation? ... Stoichiometry Basics. Stoichiometry theory of the proportions in which chemical species combine with ... – PowerPoint PPT presentation
Number of Views:110
Avg rating:3.0/5.0
Title: Material Balances on Reactive Processes
1
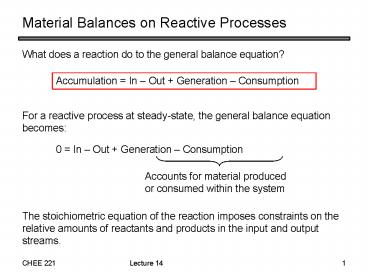
Material Balances on Reactive Processes
- What does a reaction do to the general balance
equation?
Accumulation In Out Generation Consumption
For a reactive process at steady-state, the
general balance equation becomes
0 In Out Generation Consumption
Accounts for material produced or consumed within
the system
The stoichiometric equation of the reaction
imposes constraints on the relative amounts of
reactants and products in the input and output
streams.
2
Material Balances on Reactive Processes
- What quantities are conserved??
3
Stoichiometry Basics
- Stoichiometry theory of the proportions in
which chemical species combine with one another
in a reaction - Stoichiometric Equation an equation that
relates the relative number of molecules or moles
of reactants and products (but not mass!) that
participate in a chemical reaction. To be valid,
the equation must be balanced. For example, - Are the following stoichiometric equations
balanced? - C2H5OH O2 ? CO2 H2O
- (NH4)2Cr2O7 ? Cr2O3 N2 H2O
2 SO2 O2 ? 2 SO3
4
Stoichiometry Basics
- Stoichiometric Coefficients (?i) values
preceding each molecular species (i) in a
balanced stoichiometric equation. Values are
defined to be positive for products and negative
for reactants. For the reaction, - Stoichiometric Ratio ratio of stoichiometric
coefficients in a balanced stoichiometric
equation. For the above reaction for example, - Two reactants, A and B, are in stoichiometric
proportion if the ratio (moles of A
present)/(moles of B present) equals their
stoichiometric ratio determined from the balanced
stoichiometric equation.
2 SO2 O2 ? 2 SO3
5
Limiting and Excess Reactants
- If the reactants are not present in
stoichiometric proportions, the reactant that is
completely consumed when a reaction is run to
completion is known as the limiting reactant.
The other reactant(s) are termed excess
reactant(s). - The fractional excess of the reactant is the
ratio of the excess to the stoichiometric
requirement - where, (nA)feed is the number of moles of an
excess reactant, A, present in the feed to a
reactor and (nA)stoich is the stoichiometric
requirement of A, or the amount needed to react
completely with the limiting reactant. - Percentage excess of A is 100 times the
fractional excess.
6
Identifying the Limiting Reactant
- Balance the stoichiometric equation
- Identify the reactant with the lowest
stoichiometric coefficient. If there are two
such reactants, for example, A B 2C ? D,
select the reactant with the smallest number of
moles fed. - Set up stoichiometric ratios for each reactant
using the reactant identified in Step 2 as the
denominator. - Set up corresponding ratios for each reactant
using actual feed values, using the actual feed
value of the reactant identified in Step 2 as the
denominator. - Compare the ratios ?
If,
reactant x is not the limiting reactant
If,
reactant x is the limiting reactant
7
Fractional Conversion
- Chemical reactions do not occur instantaneously,
but rather, proceed quite slowly. Therefore, it
is not practical to design a reactor for complete
conversion of the limiting reactant. Instead,
the reactant is separated from the reactor outlet
stream and recycled back to the reactor inlet.
The fractional conversion of a reactant is the
ratio of the amount reacted to the amount fed - Fractional conversion is unitless!
- The fraction unreacted (i.e., in exit stream) is
1 fA. - The percentage conversion is 100 x fA.
or
8
Extent of Reaction
- The extent of reaction is a quantity
that characterizes the reaction and simplifies
our calculations. - For a continuous process at steady-state
- where, and are the molar flow rates of
species i in the feed and outlet streams,
respectively. - For a batch process
- where, and are the initial and final
molar amounts of species i, respectively. - The extent of reaction has the same
units as n (or ).
9
Example
- Acrylonitrile is produced in the reaction of
propylene, ammonia, and oxygen - The feed contains 10.0 mole propylene, 12.0
mole ammonia, and 78.0 mole air. A fractional
conversion of 30.0 of the limiting reactant is
achieved. Taking 100 mol of feed as a basis,
determine which reactant is limiting, the
percentage by which each of the other reactants
is in excess, and the molar amounts of all
product gas constituents for a 30 conversion of
the limiting reactant.