Chapter 5 Atomic Models - PowerPoint PPT Presentation
1 / 21
Title: Chapter 5 Atomic Models
1
Chapter 5 Atomic Models
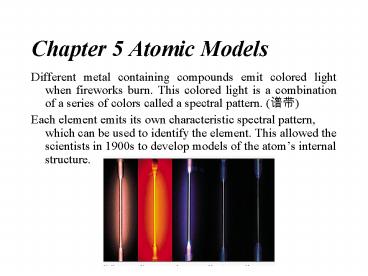
- Different metal containing compounds emit colored
light when fireworks burn. This colored light is
a combination of a series of colors called a
spectral pattern. (??) - Each element emits its own characteristic
spectral pattern, which can be used to identify
the element. This allowed the scientists in 1900s
to develop models of the atoms internal
structure.
2
5.1 Models help us visualize the invisible world
of atoms
- Atom is very small.
3
We can not see them in the usual sense. This is
because light travels in waves and atoms are
smaller than the wavelengths of visible light,
which is the light that allows the human eye to
see things. So we can not see atoms through the
media of light, even with a microscope.
4
We can see atoms indirectly through scanning
tunneling microscope (STM), which was invented in
1980s.
Fig1Scanning tunneling microscope Fig2an image
of gallium and arsenic atoms obtained with an STM
fig3 an STM image of mono-layer of perylene
derivative on graphite substrate, where the
epitaxial relationship is observed between the
organic molecule and the substrate graphite
5
5.2 Light is a form of energy
6
5.3 Atoms can be identified by the light they
emit
- When we view the light from glowing atoms, we see
that the light consists of a number of discrete
frequencies rather than a continuous spectrum.
This is called elements atomic spectrum (????).
In 1800s researchers noted the orderliness of
elements atomic spectrum, especially hydrogen,
but could not give the explanation.
7
?????
8
5.4 Niels Bohr used the quantum hypothesis to
explain atomic spectra
- Max Plancks quantum hypothesis (????) a beam of
light energy is not the continuous stream of
energy, but consists of small, discrete packets
of energy. Each packet was called a quantum. In
1905, Einstein recognized that these quanta of
light behave like particles. Each quantum was
called a photon (??). Light behaves as both a
wave and a particle.
9
Bohrs explanation
Electron loses potential energy and moves closer
to nucleus. A photon of light is emitted
Electron gains potential energy and moves farther
from nucleus. A photon of light is absorbed
10
Bohrs planetary model of atom
- There are only a limited number of permitted
energy levels in an atom, and an electron can
only stay in these energy levels. Each energy
level has a principal quantum number n (????).
The energy level with n1 has the lowest energy.
11
- Photons are emitted by atoms as electrons move
from higher-energy outer orbits to lower-energy
inner orbits. The energy of an emitted photon is
equal to the difference in energy between the two
orbits. Because an electron is restricted to
discrete orbits, only particular light
frequencies are emitted.
12
5.4 Electrons exhibit wave properties
- Question by Louis de Groglie If light has both
wave properties and particle properties, why can
not material particle, such as electron, also
have both? - Answer Every particle of matter is somehow
endowed with a wave to guide it as it travels.
The more slowly an electron moves, the more its
bahvior is that of a particle with mass. The more
quickly it moves, the more its behavior is that
of a wave of energy. - In an atom, an electron moves at very high
speeds on the order of 2000000 meters per
second. Practical application of the wave
behavior of electrons electron microscope.
13
- The electrons wave nature can be used to explain
the Bohrs planetary model (1) Permitted energy
levels are a natural consequence of electron
waves closing in on themselves in a synchronized
manner. (2) By viewing each electron orbit as a
self-reinforcing wave, we that the circumference
of the smallest orbit can be no smaller than a
single wavelength.
14
How to visualize electron waves? Probability
clouds and atomic orbitals
- In 1926, Erwin Schrodinger formulated a equation
from which the intensities of electron waves in
an atom could be calculated. It was found that
the intensity at any given location determined
the probability of finding the electron at that
location. The electron was mostly likely to be
found where its wave intensity was grestest.
15
Atomic orbitals have shapes and sizes!
16
- The size of orbital is indicated by Bohrs
principal quantum number n 1, 2, 3, 4, 5, 6, 7
Fig5.19 The fluorine atom has five overlapping
atomic orbitals that contain its nine electrons,
which are not shown
17
5.6 Energy-level diagrams describe how orbitals
are occupied
- Each orbital has a capacity of two, but no more
than two, electrons. They spin in opposite
directions, which generates two oppositely
oriented magnetic fields that are attractive and
partly compensate for the electrical repulsion
between the electrons.
18
- Example
- Lithium (3) 1s22s1
- Boron (5) 1s22s22p1
- Carbon (6) 1s22s22p2
- Nitrogen (7) 1s22s22p3
- Oxygen (8) 1s22s22p4
The properties of an atom are determined mostly
by its outermost electrons (?????), since they
are the ones in direct contact with the external
environment.
19
C 6 electrons
N 7 electrons
O 8 electrons
F 9 electrons
Ne 10 electrons
C 1s22s22p2
N 1s22s22p3
O 1s22s22p4
F 1s22s22p5
Ne 1s22s22p6
7.7
20
5.7 Orbitals of similar energies can be grouped
into shells
The seven rows correspond to the seven periods in
the periodic table. The maximum number of
electrons each row can hold is equal to the
number of elements in the corresponding period.
(2, 8, 8, 18, 18, 32, 32)
21
- From electron configuration, we can predict
- (1) The smallest atoms are at the upper right
of the periodic table. - The smallest atoms have the most strongly held
electrons. (Represented by ionization energy
(???), the amount of energy needed to pull an
electron away from an atom). The ionization
energy also determines the atoms chemical
behavior, which will be discussed in chapter 6.