LHCC PowerPoint PPT Presentation
1 / 21
Title: LHCC
1
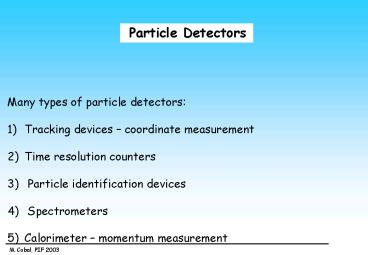
Particle Detectors
- Many types of particle detectors
- Tracking devices coordinate measurement
- Time resolution counters
- Particle identification devices
- Spectrometers
- Calorimeter momentum measurement
2
Position measurement
- Role of an Inner Tracker
- Measure charged tracks with minimal perturbation
- low mass detector ? small energy losses
- small number of primary interactions
- large fluctuations in deposited energy
- Quantities of interest
- momentum (including sign)
- angles (2) defining initial direction
- point of origin (vertex)
- Quality of data is vastly improved by magnetic
tracking - photon, electron and tau identification
- secondary vertex measurement
- isolation using charged tracks
- measuring momentum requires a magnetic field
3
- Main principle ionization products are either
visualized (as in - photoemulsion) or collected on electrodes to
produce a computer- - readable signal
- Basic requirements of high energy experiments
- high spatial resolution (100 mm)
- possibilities to register particles at the
proper moment of time - and with the high enough rate (good triggering)
- To fulfil the latter, electronic signal pick-up
is needed. This is the - reason why photoemulsion and bubble chambers
were abandoned.... - Modern tracking detectors fall in two major
categories - a) Gaseous detectors (gas chambers)
resolution 100-500 mm - b) Semiconductor detectors ( 5 mm)
4
Proportional and drift chambers
- The simplest proportional
- chamber
- A conducting chamber, filled with a gas mixture
as cathode - A wire inside serves as an anode
- Gas mixture adjustement nr of secondary
electrons caused by the - primary ionization ?to the nr of primary ion
pairs - (105 /pair for voltages of 104-105 V/cm)
- Several anode wires -gt measure coordinate
(Multi-Wire chambers)
5
- At very low voltages, charges begin to be
collected, but recombination is still - the dominant process
- Ionization mode at higher voltage, full charge
collection begins - Multiplication at a certain theshold
voltage VT the electric field close to - the surface of the anode is large enough
to begin process of - multiplication
- Proportional mode increasing V0 above VT results
in gains gt 104 with detected - charge proportional to primary deposited
charge - Limited proportionality at even higher
voltages proportionality is gradually - lost consequence of the E field
distorsions due to space - charge around the anode
- Geiger mode the region of limited
proportionality eventually ends in a - region of saturated gain same size of
signal indipendent
6
Detector gas mixtures
- Avalanche multiplication occurs in all gases
- BUT..experimental requirements limit choises
- low working voltage
- stable operation at high gain
- good proportionality
- high rate capability
- long lifetime
- fast recovery
7
- Principal component noble gas (e.g. Argon)
- allows multiplication at relatively low E field
- does not have molecules, produces only elastic
scattering (little loss of energy) - Ar gives more primary ionization than He or N
- (Kr and Xe give even more, but expensive)
- Counter full of Ar does not give stable
operations - during avalanche process many excited Ar atoms
decay emitting UV gs (11.6 eV for Ar) - UV gs strike cathod and eject photoelectrons
which gives rise to another avalanche ?
continuous discharge
8
Quenching gases
- Chamber filled with pure Ar suffers such
breakdown at low gain - Polyatomic gases have many non-radiative
vibrational and - rotational excited states over a wide energy
range - If chamber contains a fraction of such a gas, its
molecules will - absorb energy from excited argon atom by
colliding with it or - dissociating it into smaller molecules
- Since temission gtgt tcollision UV g emission is
quenched - Presence of quencing gas can give enormous
increase in - stable obtainable gain
- Common property of hydrocarbon, alcohol families
9
Alternative to MWPC drift chambers
- Ionization electrons
- produced along the
- particle passage arrive
- to the pick-up anode at
- different times
- Knowing (from other detectors) the moment of
particles arrival - and field in the chamber, one can calculate
coordinates of the track - Streamer detectors wire chambers in which
secondary ionization - is not limited and develops into moving plasmas -
streamers - If H.V. pulse is long enough, a spark will occur
which is achieved in - spark chambers
10
Semiconductor detectors
- In semiconducting materials, ionizing particles
produce electron- - hole pair, and the number of these pairs is
proportional to energy - loss by particles
- Equipping a slice of silicon with narrow pickup
conducting strips, - and subjecting it to a high voltage, one gets a
detector, analogous - to MWPC, with far better resolution
- However semiconductor detectors have rather
limited lifetimes - due to radiation damage.
11
Spectrometers
Momenta of particles are measured by the
curvature of the track in a magnetic
field Spectrometers are tracking detectors
placed inside a magnet, providing momentum
information In collider experiments, no special
spectrometers are arranged, but all the
tracking setup is contained inside a solenoidal
magnet
Scintillation counters
- To signal passage of particles through an
experimental setup and to measure the time of
flight (TOF), scintillation counters are widely
used.
12
Scintillation counters
- Scintillators are materials (crystal or organic)
in which ionizing - particles produce visible light without losing
much of its energy. - The decay times of the fastest (organic)
scintillator are 1 ns - Inorganic (i.e sodium iodide)
- Doped with activator centers. Ionizing particles
traversing the - crystal produce free electrons and holes, which
move around until - captured by an activator center. This is
transformed in an excited - state and decay with emission of light (broad
spectrum in the visible - region)
- Organic
- Mechanism is excitation of molecular levels which
decay with - emission of light in the UV
13
- The conversion of the light in the blue region is
done wia fluorescent - excitation of dye molecules known as wavelength
shifters, mixed - to the primary scintillator
- The light from the scintillator slab travels down
it by internal - reflection. At the border of the slab it is
collected by plastic light - guide or by fibres and sent to a photomultiplier
Photomultiplier Photocathode coated with alkali
metals, where electrons are liberated by
photoelectric effect Electrons travel to a chain
of secondary emission electrodes (dynodes) at
larger and Larger potentials. 4 secondary are
emitted per incident electron, amplification
factors of 108 are achieved with 14 dynodes.
Transit time 50 ns
14
Module of the Tile Hadron calorimeter of
the ATLAS experiment
Photomultiplier
Scintillating tile
15
Particle Identification
- Knowing momentum of particles is not enough to
identify them, - complementary information is needed
- For low energy particles TOF counters can
provide this - complementary data
- dE/dx depends on particle mass for energies
below 2 GeV. - Very reliable particle identification device
Cherenkov counters - In certain media, energetic charged particles
move with velocities - higher than the speed of light in these media
- Excited atoms along the path of the particle
emit coherent photons - at a characteristic angle qc to the direction of
motion
16
The angle qc depends on the refractive index of
the medium n and on the particles velocity v
cos qc c/(vn) 1/(bn) Hence,
measuring qc the velocity of the particle can be
easily derived, and the identification performed.
17
- Charged particles interact with gases, liquids,
amorphous solids - and crystals
- These interactions produce electrical or optical
signals in these - materials wich betray the particles passage
- Neutral particles are detected indirectly
through secondary - particles
- a) photons produce free electrons (Compton or
photoelectric - effects) or ee- pairs
- b) neutrons and neutrinos produce charged
particles through - interaction with nuclei
18
- Here are some of the different detectors
- Scintillators provide fast time information, but
have only moderate spatial resolution - -Gaseous counters covering large areas (wire
chambers) provide - good spatial resolution. Used in combination with
magnetic fields to - measure momentum
- Semiconductor counters have a very good energy
and spatial - resolution
- Cerenkov counters and counters based on
transition radiation - used for particle identification
- Calorimeters measure the total energy at very
high energy
19
HEP experiments
Each layer identifies and measures particles No
single detector can determine identity and
measure E and p of all particles!
20
(No Transcript)
21
LEP evts ee- -gt Z -gt ff