P2 PowerPoint PPT Presentation
Title: P2
1
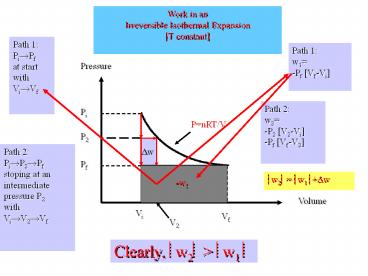
Work in an Irreversible Isothermal Expansion T
constant
Path 1 Pi?Pf at start with Vi?Vf
Path 1 w1 -Pf Vf-Vi
Pressure
Path 2 w2 -P2 V2-Vi -Pf Vf-V2
Pi
PnRT/V
P2
Path 2 Pi?P2?Pf stoping at an intermediate pressu
re P2 with Vi?V2?Vf
?w
Pf
?w2? ?w1 ??w
-w1
-w1
Volume
Vi
Vf
V2
Clearly, ?w2? gt ?w1 ?
2
Work in an Isothermal Expansion Approaching
Reversibility
Path 1 w1 -Pf Vf-Vi
Pressure
Path 1 Pi?Pf at start with Vi?Vf
Path 2 w2 -P2 V2-Vi -P3 V3-V2 ... -Pf
Vf-Vf-1
Pi
PnRT/V
Pf
-w1
-w1
Path 2 Pi?P2?P3? ?. ? Pf with Vi?V2?V3 ? ?
. ? Vf
Volume
Vi
Vf
Clearly, ?w2? gt ?w1 ?
3
Work in an Isothermal Expansion Approaching
Reversibility
Path 1 w1 -Pf Vf-Vi
Pressure
Path 1 Pi?Pf at start with Vi?Vf
Path 2 w2 -P2 V2-Vi -P3 V3-V2 ... -Pf
Vf-Vf-1
Pi
PnRT/V
Pf
-w1
Path 2 Pi?P2?P3? ?. ? Pf with Vi?V2?V3 ? ?
. ? Vf
Volume
Vi
Vf
Clearly, ?w2? gt ?w1 ?
4
True Reversible, Isothermal Expansion of an
Ideal Gas
Maximum possible work is done by a gas in a
reversible expansion.
pext pgas nRT/V
-
-wrev
5
Quantitative comparison of reversible and
irreversible work
nRT
Pex P2
and suddenly drop pressure to
v2
so fast that gas doesnt expand ?
Then expansion takes place with Pex P2
P2
P1
P
P
Reversible work
Gas
Gas
6
Bonus Bonus Bonus Bonus Bonus Bonus
7
Entropy and the Second Law
Preliminary statement There exists a state
function called entropy whose change is given by
Here dqrev is an infinitesemal change in the heat
added for a reversible process, T Kelvin
temperature.
(1) Choose a reversible path
(3) Divide dqrev by T at each point
8
If qrev were an ordinary well defined variable
(state function) like P, V could think of it
this way
for specific reversible path in qrev space
1/T
qrev
State 1
State 2
(graph is misleading because q is not an ordinary
variable!)
Thus, over any path between the same initial and
final states (reversible or irreversible) ?S has
the same value.
9
The Second Law
The simplest way to state the second law is for
an isolated system. This is a system for which
dq 0, and dw 0.
?S 0 for a reversible change
?S gt 0 for an irreversible change
(corollary ?S lt 0 is impossible for an isolated
system).
10
The word spontaneous is used interchangeably with
irreversible to describe processes that are
observed in nature to "proceed on their own".
Since we can always combine the system together
with surroundings to form an isolated system
which is the universe, we can restate the 2nd
Law
?S (universe) lt 0 impossible (never observed)
11
Entropy Calculations
1) Cardinal Rule Can only calculate entropy
change if choose a reversible path.
3) ?S for isothermal process (reversible) T
const
12
dqrev sum of heat changes over path. Total
is qrev or heat added going from state 1 to 2
over reversible path.
For an ideal monatomic gas E (3/2) RT
?E q w 0 ? w - q
In a reversible process, PPext and V is not
constant.
13
?S nR ln (V2 / V1 ) Ideal gas isothermal
change.
Note this last case is NOT inconsistent with the
2nd law!
System entropy can decrease as long as entropy of
universe either increases or stays the same. If
system entropy decreases, entropy of surroundings
must increase.
As we shall see later, the fact that we need to
include both the system and surroundings when
considering the Second Law makes the ?S function
inconvenient.
14
Temperature Dependence of Entropy
For constant pressure process
For infinitesimal change dqrev nCp dT Now
assume Cp independent of T
?Sp nCp ln (T2 / T1)
Assumes Cp , Cv are constant over range T1 ? T2
15
For an ideal gas Cp (3/2)R R (5/2)R
Note that ?Sp , ?Sv are not the same. Cannot be
between same initial and final states! (T1, T2
can be same but P1, P2 and V1, V2 must be
different).
Absolute Entropies and the Third Law
In same way can set up an arbitrary scale for
?Sf?. As it turns out, can also set up an
absolute scale for entropy.
16
Third Law of Thermodynamics
dqrevCpdT
But ?S ? dqrev/T
Note Really cannot take Cp independent of T here
because are going from absolute zero to some
finite T. Most substances go from solid to
liquid to gas over this range!
S298 ? Standard absolute entropy TF 298 K and
P 1 atm.