Lect 7Thermal oxidization - PowerPoint PPT Presentation
1 / 15
Title:
Lect 7Thermal oxidization
Description:
Upon exposure to oxygen, the surface of the silicon wafer is oxidized and a thin ... SiO2 works as a perfect insulator as well as ... T is temperature in Kelvin ... – PowerPoint PPT presentation
Number of Views:60
Avg rating:3.0/5.0
Title: Lect 7Thermal oxidization
1
Lect 7 Thermal oxidization
- Upon exposure to oxygen, the surface of the
silicon wafer is oxidized and a thin layer of
silicon dioxide is formed. - SiO2 works as a perfect insulator as well as a
barrier layer. - Thermal oxidization is a process where the
silicon wafer is heated to high temperatures (900
to 1200o) in the presence of oxygen or water. - The interaction between O2 (H2O) and Si results
in an oxide layerSi O2 ? SiO2 Si H2O ?
SiO2 2H - At these high temperatures O2 and H2O vapor
diffuses easily through the silicon dioxide
layer and interacts with the silicon beneath to
further expand the oxide layer. - The diffusion of different molecules is
charac-terized by the diffusion coefficient, D,
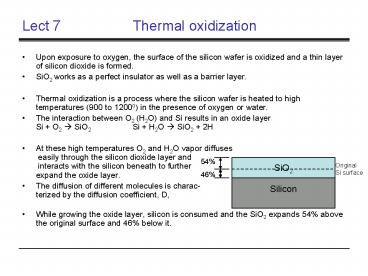
- While growing the oxide layer, silicon is
consumed and the SiO2 expands 54 above the
original surface and 46 below it.
Original Si surface
2
Thermal oxidization
Original layer of oxide
- Due to the exposure of the surface to oxygen
andwater (oxidizers), there exists an initial
layer of oxide of thickness Xi. - When heating the wafer at high temperatures
theoxidizers diffuse through the SiO2 and
oxidize the silicon layer beneath - As the oxide layer grows, the oxidizer needs to
gothrough more and more of SiO2 layers causing
theoxidization rate to reduce.
Silicon
Heating with the presence of O2 or H2O
Silicon
SiO2
Newly oxidized layer
Si
Heating with the presence of O2 or H2O
Silicon
3
Thermal oxidization
- To model this process we will assume that oxygen
diffuses through SiO2. - Ficks first law of diffusion Particles flow per
unit area, J (referred to as flux) is
proportional to the concentration gradient. - N is the concentration of oxidizers inside the
SiO2 number of oxidizer per unit volume. - Assume that the flux is constant through the SiO2
layer as no oxidizers are consumed. Then - The boundary condition is that, at x0, NNO,
where NO is the concentration of the oxidizers at
the oxide surface. At the Si-SiO2 interface, xXO
and the concentration is Ni, then - At the Si-SiO2 interface, the oxidizers react
with Si creating SiO2. Hence, the flux is assumed
to be proportional to the concentration Ni - ks is the reaction rate constant.
4
Thermal oxidization
- The flux, J, of the oxidizers through the SiO2 is
defined as number of particles per unit area per
unit time. - Assume that M is the molecules density of the
oxidizers which incorporated into a unit volume
of the resulting oxide - Then the total number of particles that oxidize a
unit area A and thickness dX0 is - The total number of particles per A per unit time
dt is - Replacing the flux with the expression from
before - Integrating both sides
No
Concentration N
Ni
SiO2 Si
Xo
Distance from surface, x
SiO2
5
Lect8 Thermal oxidization
- The integration constant can be estimated
applying the proper boundary conditions - Boundary conditions t0, X0Xi
- Deal-Groove model
- The solution to this equation is the
- If then
if
and
6
Thermal oxidization
- For very short time, the thickness changes
linearly with time, hence B/A is referred to as
the linear rate costant - For very long time, the thickness changes in
quadratic form with time, hence B is referred to
as the parabolic rate constant - Both constant change with temperaturein an
exponential form. - D can be either B/A or BDo is a constant EA is
referred to as the activation energyk is
Boltzmanns constant (1.381x10-23 J/K)T is
temperature in Kelvin - The equation indicates a strong dependence of the
oxide thickness on temperature
Xo(t)
Linear region
Parabolic region
Xi
t
7
Thermal oxidization
- Typical values for Co and EA are in the table
below - The table shows a finite value for Xi for the dry
O2 case. That indicates that a finite value of ??
is needed. - The large value of Xi (25 nm) indicates some sort
of inaccuracy in the model.
8
Thermal oxidization
- The oxide growth rate is much higher in wet
atmosphere. - Hence, larger thickness when using wet O2
compared to dry O2. - Slow growth rate results in dense and high
quality oxide and hence it is usually used for
the MOS gate oxide region. - Fast growth rate in wet oxygen results in a lower
quality oxide region and it is used for as a
masking or protection layer. - From the equations both rate constants A and B
depend on No which depends on the pressure of the
oxidizing species.
9
Thermal oxidization
- The diffusion coefficients of several impurities
(Boron, Phosphorous) in SiO2 are orders of
magnitudes smaller than in Si. - That qualifies SiO2 as a mask for impurities
during high temperature diffusion. - Relatively deep diffusion can take place in
unprotected regions of silicon whereas no
significant impurities penetrate through the
covered regions. - Typical values of Do for Boron and Phosphorous in
SiO2 at 1100 oC are - Typical values of Do for Boron and Phosphorous in
Si at 1100 oC are - The presence of ambient gases (impurities) in the
substrate (Si or SiO2) influences diffusion.
10
Thermal oxidization
- Oxidation technology
- Most thermal oxidation is performed in furnaces,
at temperatures between 800 and 1200C. - A single furnace accepts many wafers at the same
time, in a specially designed quartz rack (called
a "boat"). - Historically, the boat entered the oxidation
chamber from the side (this design is called
"horizontal"), and held the wafers vertically,
beside each other. - Many modern designs hold the wafers horizontally,
above and below each other, and load them into
the oxidation chamber from below. - Vertical furnaces stand higher than horizontal
furnaces, so they may not fit into some
microfabrication facilities. However, they help
to prevent dust contamination. Unlike horizontal
furnaces, in which falling dust can contaminate
any wafer, vertical furnaces only allow it to
fall on the top wafer in the boat.
11
Lect. 9 Thermal Oxidation
- Oxide quality
- Contamination metal ions such as sodium are
highly mobile in SiO2 Mobile and can degrade the
performance of the MOSFET.- Sodium-ions cause
positive charges in the oxide and an excessive
positive charge at the Si-SiO2 interface. -These
charges attract electrons to the surface of the
MOS causing a negative shift in the threshold
voltages.-chlorine can immobilize sodium by
forming sodium chloride. Chlorine is often
introduced by adding hydrogen chloride or
trichloroethylene to the oxidizing medium. Its
presence also increases the rate of oxidation? - Dirty interface Growing thick oxides using wet
oxygen the higher growth rate leaves more
dangling bonds at the silicon interface, which
produce quantum states for electrons and allow
current to leak along the interface. - Density Wet oxidation also yields a
lower-density oxide, with lower dielectric
strength. - Breakdown voltage Dry oxidization with higher
density results in high breakdown voltage.
However, the long time required to grow a thick
oxide in dry oxygen makes this process
impractical. Thick oxides are usually grown with
a long wet oxidation bracketed by short dry ones
(a dry-wet-dry cycle). The beginning and ending
dry oxidations produce films of high-quality
oxide at the outer and inner surfaces of the
oxide layer, respectively.
12
Thermal oxidation
- Local oxidation
- The ability to selectively oxide specific
locations on the silicon wafer is of great
interest in high density transistor processes. - The technique utilized for localized oxidation is
referred to as LOCOS (Local Oxidation Of
Silicon). - To perform local oxidation, the areas not meant
to be oxidized will be coated in a material that
does not permit the diffusion of oxygen. - Oxygen and water vapor do not diffuse well
through silicon nitride, hence Si3N4 is used as
an oxidization barrier. - For local oxidization process -a thin layer of
SiO2 is deposited to protect the wafer -A Si3N4
is deposited over the oxide.-A photolithography
step is applied and a pattern is etched through
the two layers.
Si3N4 SiO2
Silicon wafer
13
Thermal oxidation
- Semirecessed oxide structure
Fully-recessed oxide structure
Silicon wafer
Silicon wafer
Silicon etching
Oxidation
Oxidation
Nitride removal
Nitride removal
Almost flat oxide surface
14
Thermal oxidation
- Oxide thickness characterization
- The simplest way to characterize the thickness of
an oxide is by comparing the color of the wafer
with the reference color chart attached. - When light transmits through SiO2, reflects from
theSiO2-Si interface and leaves the oxide it
gains a totalphase of and an
amplitude of r. - The total reflected field is a summation of the
infinite reflections - The total field forms a power series.
- The intensity is
Reflected light
Incident light
.
.
d
SiO2
Silicon
15
Thermal oxidation
- The reflected light intensity is
- The peak of the intensity when??2? m, where m is
integer. - At a certain peak of wavelength ?? the reflected
light will appear with a color that corresponds
to that wavelength. - Hence the color can be translated directly into
a thickness shown by the equation above and the
chart table attached.