Isotopes PowerPoint PPT Presentation
1 / 24
Title: Isotopes
1
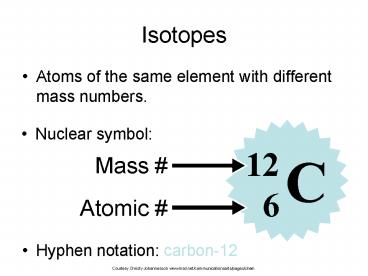
Isotopes
- Atoms of the same element with different mass
numbers.
- Nuclear symbol
- Hyphen notation carbon-12
Courtesy Christy Johannesson www.nisd.net/communic
ationsarts/pages/chem
2
Isotopes
Electrons
Nucleus
Electrons
Neutrons 6 Protons 6 Electrons 6
Neutrons 8 Protons 6 Electrons 6
Nucleus
3
Isotopes
- Chlorine-37
- atomic
- mass
- of protons
- of electrons
- of neutrons
- 17
- 37
- 17
- 17
- 20
Courtesy Christy Johannesson www.nisd.net/communic
ationsarts/pages/chem
4
Relative Atomic Mass
- 12C atom 1.992 10-23 g
- atomic mass unit (amu)
- 1 amu 1/12 the mass of a 12C atom
Neutron
- 1 p 1.007276 amu1 n 1.008665 amu1 e-
0.0005486 amu
Electrons
Nucleus
Proton
Nucleus
Carbon-12
Neutrons 6 Protons 6 Electrons 6
5
Average Atomic Mass
- weighted average of all isotopes
- on the Periodic Table
- round to 2 decimal places
Courtesy Christy Johannesson www.nisd.net/communic
ationsarts/pages/chem
6
Average Atomic Mass
- EX Calculate the avg. atomic mass of oxygen if
its abundance in nature is 99.76 16O, 0.04 17O,
and 0.20 18O.
16.00 amu
Courtesy Christy Johannesson www.nisd.net/communic
ationsarts/pages/chem
7
Average Atomic Mass
- EX Find chlorines average atomic mass
if approximately 8 of every 10 atoms are
chlorine-35 and 2 are chlorine-37.
35.40 amu
Courtesy Christy Johannesson www.nisd.net/communic
ationsarts/pages/chem
8
Isotopes
- Dalton was wrong.
- Atoms of the same element can have different
numbers of neutrons - different mass numbers
- called isotopes
C-12 vs. C-14
California WEB
9
Naming Isotopes
- Put the mass number after the name of the element
- carbon- 12
- carbon -14
- uranium-235
California WEB
10
Atomic Number and Mass Number
Atomic Number and Mass Number
Atomic Number and Mass Number
11
Ions and Subatomic Particles
Ions and Subatomic Particles
Ions and Subatomic Particles
12
Atomic Mass
- How heavy is an atom of oxygen?
- There are different kinds of oxygen atoms.
- More concerned with average atomic mass.
- Based on abundance of each element in nature.
- Dont use grams because the numbers would be too
small
California WEB
13
Measuring Atomic Mass
- Unit is the Atomic Mass Unit (amu)
- One twelfth the mass of a carbon-12 atom.
- Each isotope has its own atomic mass we need the
average from percent abundance.
California WEB
14
Calculating averages
- You have five rocks, four with a mass of 50 g,
and one with a mass of 60 g. What is the average
mass of the rocks? - Total mass (4 x 50) (1 x 60) 260 g
- Average mass (4 x 50) (1 x 60) 260 g
5 5 - Average mass 4 x 50 1 x 60 260 g
5 5 5
California WEB
15
Calculating averages
- Average mass 4 x 50 1 x 60 260 g 5
5 5 - Average mass .8 x 50 .2 x 60
- 80 of the rocks were 50 grams
- 20 of the rocks were 60 grams
- Average as decimal x mass as
decimal x mass as decimal x mass
California WEB
16
Atomic Mass
- Calculate the atomic mass of copper if copper has
two isotopes. 69.1 has a mass of 62.93 amu and
the rest has a mass of 64.93 amu.
California WEB
17
Isotopes
- Because of the existence of isotopes, the mass of
a collection of atoms has an average value. - Average mass ATOMIC WEIGHT
- Boron is 20 B-10 and 80 B-11.
That is, B-11 is 80 percent abundant on
earth. - For boron atomic weight
- 0.20 (10 amu) 0.80 (11 amu) 10.8 amu
18
Periodic Table
- Dmitri Mendeleev developed the modern periodic
table. - Argued that element properties are periodic
functions of their atomic weights. - We now know that element properties are
periodic functions of their
ATOMIC NUMBERS.
19
Atomic Mass
- Magnesium has three isotopes. 78.99 magnesium 24
with a mass of 23.9850 amu, 10.00 magnesium 25
with a mass of 24.9858 amu, and the rest
magnesium 26 with a mass of 25.9826 amu. What is
the atomic mass of magnesium? - If not told otherwise,
- the mass of the isotope is the mass number
in amu.
24.304 amu
California WEB
20
Atomic Mass
- Calculate the atomic mass of copper if copper has
two isotopes. 69.1 has a mass of 62.93 amu and
the rest has a mass of 64.93 amu.
21
- B. C.
- D. E. F.
- G. H. I.
Given the average atomic mass of an element is
118.21 amu and it has three isotopes (A, B,
and C) isotope A has a mass of 117.93 amu
and is 87.14 abundant isotope B has a mass
of 120.12 amu and is 12.36 abundant Find the
mass of isotope C. Show work for credit.
Extra Credit What is a cation?
22
Given the average atomic mass of an element is
118.21 amu and it has three isotopes (A, B,
and C) isotope A has a mass of 117.93 amu
and is 87.14 abundant isotope B has a mass
of 120.12 amu and is 12.36 abundant Find the
mass of isotope C. Show work for credit.
119.7932 amu
Extra Credit What is a cation?
A positively charged atom. An atom that has lost
a(n) electron(s).
23
Given the average atomic mass of an element is
118.21 amu and it has three isotopes (A, B,
and C) isotope A has a mass of 117.93 amu
and is 87.14 abundant isotope B has a mass
of 120.12 amu and is 12.36 abundant Find the
mass of isotope C. Show work for credit.
24
Isotopes
Isotopes Lab
Isotopes Lab