Advanced Organic Chemistry - PowerPoint PPT Presentation
1 / 17
Title:
Advanced Organic Chemistry
Description:
Hydroxy-directed and Diastereoselective Epoxidation. Allylic Conformation and Strain ... hydroxy-directed. peracid epoxidation. transition-metal. mediated ... – PowerPoint PPT presentation
Number of Views:144
Avg rating:3.0/5.0
Title: Advanced Organic Chemistry
1
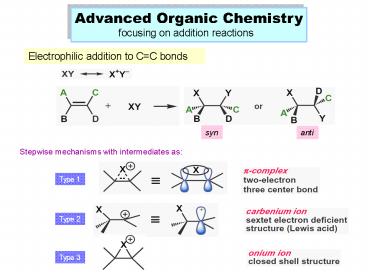
Advanced Organic Chemistry focusing on addition
reactions
Electrophilic addition to CC bonds
syn
anti
Stepwise mechanisms with intermediates as
Type 1
Type 2
Type 3
2
Type 1
Type 2
carbenium ion stability hyperconjugation
Wagner-Meerwein shift
3
anti
syn
Type 1
Type 2
4
Syn addition
Q1
tight ion-pair very stable because of
benzylic-type
Cf. solvent-separated ion-pair
5
Mechanistic Rationale
6
Peroxide Effects
no air, 25 ?, 240 h
Markovnikov rule
air, 25 ?, lt 16 h
R-O-O-R
2 R-O?
R-O?
HBr
R-OH
Br?
Br?
HBr
Br?
O2
R-H
R?
H-OO?
Q2
R?
O2
R-OO?
R-OO?
R?
R-OO-R
R-O-O-R
7
Addition of Br2 to CC Double Bonds
electrophilic, anti, stereospecific
Br3- as nucleophile
Bromonium ion Type-3
anti
Type-1
8
Physical Evidence for Bromine-Olefin
p-complex and Bromonium Ion
9
Reaction Pathway Switching low Br2
concentration, Wohl-Ziegler bromination
NBS
Free radical initiator
radical chain
10
Trapping of Bromonium Ions with Nucleophiles
other than Br-
regioselective ring-opening
Bromine syn-addition
Q3
11
Electrophilic Addition to Alkynes
Type-A
Type-B
Type-C
Linear unstable
Markovnikov rule
12
Addition of Br2 to Carbon-Carbon Triple Bonds
Synthesis of trans-1,2-dibromo Olefins
Vinylic SN1 Reaction cation-stabilizing groups
or good leaving groups
13
Electrophilic Addition of Peracids or
Hydroperoxides to CC Bonds
concerted
Bartlett 1957
Q4
Nucleophilic or 1,3-dipolar mechanism ?
Relative rates with peracid at 25.7 ?
14
Epoxidation of Allylic Alcohols with
Hydroperoxide
Regeneration of catalyst
t-BuOH
trigonal bypiramidal
product
trans-ligand effect
ROH
15
Hydroxy-directed and Diastereoselective
Epoxidation
16
Allylic Conformation and Strain
most stable
Allylic Strain A1,3
3
2
1
Allylic Conformation
17
Stereochemical Outcomes of two Representative
Epoxidations
transition-metal mediated t-BuOOH epoxidation
hydroxy-directed peracid epoxidation