Childhood Cancer Survival Trends PowerPoint PPT Presentation
Title: Childhood Cancer Survival Trends
1
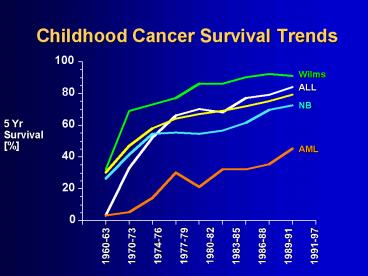
Childhood Cancer Survival Trends
5 Yr Survival
1974-76
1977-79
1980-82
1960-63
1970-73
1983-85
1986-88
1989-91
1991-97
2
High-Risk Neuroblastoma 1978 - 1995
1
0.8
0.6
Probability of Overall Survival
0.4
0.2
1978-1985 N507
1
2
3
4
5
6
7
8
9
10
Years from Diagnosis
3
Rhabdomyosarcoma Treatment
4
Doxorubicin Cardiotoxicity
Risk of CHF
Risk of CHF
Cumulative anthracycline dose mg/m2
Time from start of anthracyline therapy yr
Kremer et al JCO 2001
5
Pediatric Phase 1 Trial of Gleevec
- Phase 1 trial
- Recommended dose
- PK
- Response
- Potential targets
- bcr-abl
- PDGF-R
- c-kit
- Ph Leukemias
- Osteosarcoma
- Synovial sarcoma
- Ewings sarcoma
- Desmoplastic sarcoma
- AML
- GIST
6
Criteria for Pediatric Phase 1 Study
- Availability of new agent for pediatric studies
- Relevance of drug target in pediatric
malignancies - Activity in pre-clinical model systems
- Experience in adult clinical trials
7
Initiating Pediatric Phase 1 Trials
Time
Adult Trials
Phase 1
Phase 2
Phase 3
Phase 4
Phase 1
Pediatric Trials
Phase 1
Phase 1
Phase 1
8
Limitations of Current Approach
- Historically, patient numbers were rate limiting
step for phase 1 trials. - Currently, insufficient number of new agents are
in pediatric phase 1trials - Phase 1 trials initiated following drug approval
for adults results in use in children without any
pharmacologic, safety or efficacy data
9
COG Phase 1 Consortium
10
Current COG Phase 1 Trials
- Solid Tumors
- PS-341
- ZD1839
- Flavopiridol
- Neuroblastoma
- Hu14.18-IL2
- Select CNS Tumors
- Gadolinium-Texaphyrin
- Temozolomide/CCNU
- Cereport/Carboplatin
- Hematologic
- Arsenic Trioxide
- R115777
- IDEC-Y2B8
Dose levels fill in lt 15 minutes Necessitated
development of waiting lists
11
Recommendations
- Improve early access to new agents for
pre-clinical studies - Initiate phase 1 trials of select agents when
- Initial cohort(s) of adult patients in phase 1
are evaluable - Evidence of biologic activity observed
12
(No Transcript)
13
(No Transcript)
14
(No Transcript)
PowerShow.com is a leading presentation sharing website. It has millions of presentations already uploaded and available with 1,000s more being uploaded by its users every day. Whatever your area of interest, here you’ll be able to find and view presentations you’ll love and possibly download. And, best of all, it is completely free and easy to use.
You might even have a presentation you’d like to share with others. If so, just upload it to PowerShow.com. We’ll convert it to an HTML5 slideshow that includes all the media types you’ve already added: audio, video, music, pictures, animations and transition effects. Then you can share it with your target audience as well as PowerShow.com’s millions of monthly visitors. And, again, it’s all free.
About the Developers
PowerShow.com is brought to you by CrystalGraphics, the award-winning developer and market-leading publisher of rich-media enhancement products for presentations. Our product offerings include millions of PowerPoint templates, diagrams, animated 3D characters and more.