GC PowerPoint PPT Presentation
Title: GC
1
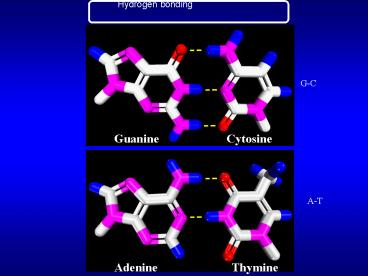
Hydrogen bonding
G-C
Guanine
Cytosine
A-T
Adenine
Thymine
2
How much is a hydrogen bond worth? experimental
value is extremely variable values in the
literature from 0.1 to 40 kcal/mol
Strength of an H-bond is related to the D-H---A
distance and the DHA angle. The shorter the
distance between D A the stronger the
interaction. Ideally, DHA angle should be 180.
3
Hydrogen bonding
If we calculate the energy of H-bond in
chloroform (? 4.8) to be 10 kcal/mol, and then
change solvent to water (? 78.8) all else being
the same we would expect the new energy of
interaction to be 10 . 4.8 / 78.8 0.6 kcal/mol
based on our equation for dipole-dipole
interactions. However, we find experimentally
that the H-bond is only worth 0.1 kcal/mol. Why
might this be?
R
2
O
H
4
Hydrogen bonding in water
ST2 Model of water molecule
5
Crystal Structure of Ice
6
(No Transcript)
7
Hydrogen Bonding
8
(No Transcript)
9
Hydrogen bonding in supramolecular synthesis
10
Hydrogen bonding in supramolecular synthesis
11
Hydrogen bonding in supramolecular synthesis
PowerShow.com is a leading presentation sharing website. It has millions of presentations already uploaded and available with 1,000s more being uploaded by its users every day. Whatever your area of interest, here you’ll be able to find and view presentations you’ll love and possibly download. And, best of all, it is completely free and easy to use.
You might even have a presentation you’d like to share with others. If so, just upload it to PowerShow.com. We’ll convert it to an HTML5 slideshow that includes all the media types you’ve already added: audio, video, music, pictures, animations and transition effects. Then you can share it with your target audience as well as PowerShow.com’s millions of monthly visitors. And, again, it’s all free.
About the Developers
PowerShow.com is brought to you by CrystalGraphics, the award-winning developer and market-leading publisher of rich-media enhancement products for presentations. Our product offerings include millions of PowerPoint templates, diagrams, animated 3D characters and more.