Kinetic and Thermodynamic Stability - PowerPoint PPT Presentation
1 / 1
Title:
Kinetic and Thermodynamic Stability
Description:
1. 1. Paramagnetic sample: drawn towards the higher field ... If we ignore the orbital contribution, there is a simple formula relating to n (the # of upe) ... – PowerPoint PPT presentation
Number of Views:381
Avg rating:3.0/5.0
Title: Kinetic and Thermodynamic Stability
1
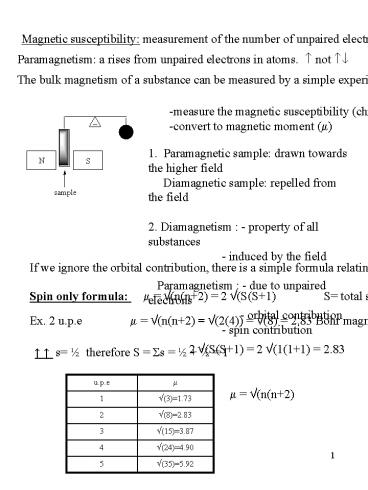
Magnetic susceptibility measurement of the
number of unpaired electrons.
Paramagnetism a rises from unpaired electrons in
atoms. ? not ??
The bulk magnetism of a substance can be measured
by a simple experiment Gooy
-measure the magnetic susceptibility (chi,
?) -convert to magnetic moment (?)
1. Paramagnetic sample drawn towards the higher
field Diamagnetic sample repelled from the
field 2. Diamagnetism - property of all
substances - induced by the field
Paramagnetism - due to unpaired electrons
- orbital contribution
- spin contribution
If we ignore the orbital contribution, there is a
simple formula relating ? to n (the of upe)
Spin only formula ? ?(n(n2) 2
?(S(S1) S total spin
Ex. 2 u.p.e ? ?(n(n2) ?(2(4)) ?(8) 2.83
Bohr magnetons.
2 ?(S(S1) 2 ?(1(11) 2.83
? ? s ½ therefore S Ss ½ ½ 1
? ?(n(n2)