Balancing Reactions PowerPoint PPT Presentation
1 / 30
Title: Balancing Reactions
1
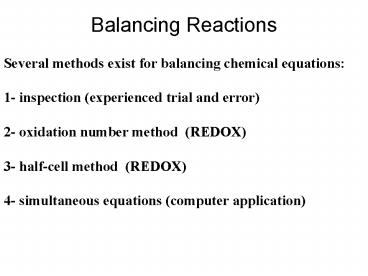
Balancing Reactions
Several methods exist for balancing chemical
equations 1- inspection (experienced trial and
error) 2- oxidation number method (REDOX) 3-
half-cell method (REDOX) 4- simultaneous
equations (computer application)
2
Balancing redox reactions
- Identify half reactions
- Balance all atoms but O, H
- Balance the charge
- Multiply so both half reactions share the same
of electrons - Balance O and H by adding OH-, H or H2O
- Add the half reactions together
- Cancel like terms on both sides
3
Balancing Redox Equations
- gt Half-reaction technique
- Examples
- Sn2 Fe3 ? Sn4 Fe2
- Fe2 MnO4- ? Fe3 Mn2 (in acid)
4
Sn2 Fe3 ? Sn4 Fe2
- Sn2 ? Sn4 2 e-
- e- Fe3 ? Fe2
- 2(e- Fe3 ? Fe2)
- --------------------------------------------------
-- - Sn2 2 Fe3 ? Sn4 2 Fe2
Check Atoms Sn 1
1 Fe 2
2 Charge
8 8
5
Fe2 MnO4- ?Fe3 Mn2
- MnO4- ? e- ? Mn2
- oxidation numbers -1 1(x)Mn 4(-2)O 2
- x 8 - 1 7
- MnO4- ? e- ? Mn2
- 7 2
- MnO4- 5 e- ? Mn2
- MnO4- 5 e- ? Mn2 4H2O
- MnO4- 8 H 5 e- ? Mn2 4H2O
6
Fe2 MnO4- ?Fe3 Mn2
- MnO4- 8 H 5 e- ? Mn2 4H2O
- Fe2 ? Fe3 e-
- 5(Fe2 ? Fe3 e-)
- ----------------------------------------
- 5Fe2 MnO4- 8H ? 5Fe3 Mn2 4H2O
Check Atoms Fe 5
5 Mn 1
1 O
4 4 H
8 8 Charge
5(2) -1 8(1) 5(3) 2(1)
17
17
7
Your turn
- Cu (s) NO3- (aq) ? Cu2 (aq) NO (g) in acid
Oxidation half-cell Cu ? Cu2
2 e-
5 -2 2 -2 Reduction
half-cell NO3- 3e- ? NO
4 H NO3- 3 e- ? NO 2 H2O
Balance electron exchange 3(Cu ? Cu2 2 e-)
2(4 H NO3- 3 e- ? NO
2 H2O)
--------------------------------------------------
-----------------------------
3 Cu 8 H 2 NO3- ? 3 Cu2 2 NO 4 H2O
Check Atoms Cu 3
3 H
8 8
N 2
2 O 6
6 Charge
6 6
8
How to determine if electrons have transferred
- Oxidation Numbers
- Assign electrons to the most electronegative
element in a bond. The oxidation number is the
charge an atom would have if the compound were
completely composed of ions. - RECORD KEEPING.
9
Oxidation Numbers Useful Method
- Oxidation numbers are an electron count
bookkeeping scheme. - When a compound is formed, the electrons
distribute themselves, according to
electronegativity differences - Metals tend to give up electrons (oxidation)
- gt becoming positive
- Non-metals tend to receive electrons (reduction)
- gt becoming negative
It is useful to have a method for keeping track
of the electrons
10
Oxidation Numbers
- Rules for assigning oxidation numbers
- 1- treat ions separately
- 2- sum of oxidation numbers on species (molecule,
ion) is equal to the charge - 3- Hydrogen usually has ox no 1
- exception hydride, -1
- 4- Oxygen usually has ox no -2
- exceptions peroxide, -1 superoxide,
-1/2 - 5- halogens usually are -1 (F, Cl, Br, I)
- 6- most electronegative atom often has ox no
equal to number of electrons needed to complete
its octet
11
Rules for Determining Oxidation States
- Oxygen has a -2 oxidation state in its covalent
compounds - EXO in CO, CO2, SO2, SO3
- Exception oxygen has a -1 oxidation state in
peroxides such as H2O2
12
Rules for Assigning Oxidation State
- Any element in its free state has an oxidation
state of zero - EXmetals Cu, Ag, Hgnon-metals H2, O2, He,
Ne, etc.
13
Rules for Assigning Oxidation States
- In binary compounds (AaBb), the element with the
greater electronegativity is assigned the
negative oxidation state - EXNH3 (N is -3)H2S (S is -2)
14
Rules for Assigning Oxidation States
- The oxidation state on a monatomic ion is its
charge which can be determined by the position of
the element in the Periodic Table - EXNa, Mg2, F-, Cl-
15
Rules for Assigning Oxidation States
- The sum of the oxidation states of all atoms in a
compound is zero - EXKMnO4NaCl
16
Rules for Assigning Oxidation States
- The sum of the oxidation states of all atoms in a
polyatomic ion is the charge on the ion - EXPO43-ClO4-
17
Oxidation Numbers
- Examples with a strategy
- NH4NO3 NH4 NO3- treat
separately - Ox no for each element x 1
-3 1 - N H4
? N H4 - Sum of ox numbers x 4(1) 1
- x
4 1 x -3 - Ox no for each element x -3
5 -2 - N O3-
? N O3- - Sum of ox numbers x 3(-2) -1
- x -
6 -1 x 5
18
Oxidation Numbers
- Examples with a strategy
- FeO and
Fe2O3 - Ox no for each element x -2
2 -2 - FeO
? FeO - Sum of ox numbers x (-2) 0
- x -
2 0 x 2 - Ox no for each element x -2
3 -2 - Fe2O3
? Fe2O3 - Sum of ox numbers 2(x) 3(-2) 0
- 2x
- 6 0 x 3 - Same element exists in three different oxidation
states! Fe 0, 2, 3 - Others Sn 0, 2, 4 N 0, -3, 1, 2, 3,
4, 5 Cl 0, -1, 1, 3, 5, 7
19
(No Transcript)
20
Oxidation Numbers
- Examples with a strategy
- FeO (s) CO (g) ? Fe (s) CO2 (g)
Fe gains two electrons
C gives up two electrons
In a balanced REDOX equation Number of
electrons received Number of electrons given
up Number electron in reduction Number
electrons in oxidation
21
(No Transcript)
22
(No Transcript)
23
check the oxygen balance
24
(No Transcript)
25
(No Transcript)
26
Problem 1 and 2
- Determine the oxidation number of each atom in
the following species - BaCl2
- LiH
- Zn
- O2
- H2CO3
- Cr2O72-
- Determine if the following changes represents an
oxidation or a reduction - MnO2 to Mn2O3
- Pb(OH)42- to PbO2
27
(No Transcript)
28
Anode Pb grid
Cathode Pb grid filled w/ PbO2
H2SO4 electrolyte
-0.3588
E Ecat Ean 1.69 - (-0.3588) 2.0488V
Spontaneous?
29
-0.7618
30
(No Transcript)