Diagnosis of a benzene discharge with a mass-selective spectroscopic technique
1 / 1
Title:
Diagnosis of a benzene discharge with a mass-selective spectroscopic technique
Description:
Institute of Physical Chemistry, University of Basel, Klingelbergstrasse 80, CH ... 5) C. Lifshitz, G. Reuben, J. Chem. Phys. 50, 951 (1969). Acknowledgment ... –
Number of Views:48
Avg rating:3.0/5.0
Title: Diagnosis of a benzene discharge with a mass-selective spectroscopic technique
1
Diagnosis of a benzene discharge with a
mass-selective spectroscopic technique
Felix Güthe, Hongbin Ding, Thomas Pino and John
P. Maier Institute of Physical Chemistry,
University of Basel, Klingelbergstrasse 80,
CH-4056 Basel, Switzerland.
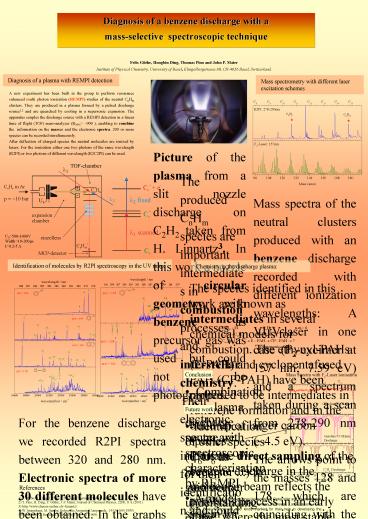
Diagnosis of a plasma with REMPI detection
Mass spectrometry with different laser excitation
schemes
A new experiment has been built in the group to
perform resonance enhanced multi photon
ionization (REMPI) studies of the neutral CnHm
clusters. They are produced in a plasma formed by
a pulsed discharge source1,2 and are quenched by
cooling in a supersonic expansion. The apparatus
couples the discharge source with a REMPI
detection in a linear time of flight (TOF)
mass-analyzer (R50 900 ), enabling to combine
the information on the masses and the electronic
spectra. 200 or more species can be recorded
simultaneously. After deflection of charged
species the neutral molecules are ionized by
lasers. For the ionization either one two photons
of the same wavelength (R2PI) or two photons of
different wavelength (R2C2PI) can be used.
Picture of the plasma from a slit nozzle
discharge on C2H2 taken from H. Linnartz3. In
this work a nozzle of circular geometry with
benzene as precursor gas was used but could not
be photographed.
The produced CnHm species are important
intermediates in combustion processes and
interstellar chemistry. Their electronic spectra
are important for their identification and could
be related to the diffuse interstellar band
problem.
Mass spectra of the neutral clusters produced
with an benzene discharge recorded with different
ionization wavelengths A VUV-laser in one case
(F2-excimer at 157 nm 7.59 eV) and a spectrum
taken during a scan from 278-290 nm (4.5
eV). The arrows point to the masses 128 and 178,
which are coinciding with the masses of the first
members of the polycyclic aromatic hydrocarbons
(PAH). But only the electronic spectra can reveal
the identity of the carriers of this mass peaks !
U0500-1000V Width10-200ms I0.2-5 A
Identification of molecules by R2PI spectroscopy
in the UV range
Chemistry in the discharge plasma
The species identified in this work are known as
intermediates in several chemical models for
combustion. The ethynyl-PAHs (E-PAH) and
cyclopentafused PAH (CP-PAH) have been proposed
to be intermediates in fullerene formation and in
the build up of larger carbon species. Thus the
direct sampling of the benzene discharge in the
molecular beam reflects the pyrolysis process in
an early stage, where the most stable isomers
have not yet been formed. Their formation might
occur at later stage at higher temperature4. The
formation of the neutral species seen in the
spectra can be assumed to occur by two stages in
analogy to cation chemistry known from electron
impact work in high pressure sources5
Conclusion
- Combination of a plasma discharge source with
spectroscopic characterisation by REMPI. - -A model system for hydrocarbon flames??
Future work
For the benzene discharge we recorded R2PI
spectra between 320 and 280 nm. Electronic
spectra of more 30 different molecules have been
obtained. In the graphs the spectra of
phenylacetylene (C8H6), styrene (C8H8), indene
(C9H8), methylstyrene (C9H10), fluorene (C13H10),
tolane (C14H10) as well as the of molecule C10H8
are shown. From these 6 molecules could be
identified by there spectra from literature.
Note that the spectrum of . C14H10 is not that of
anthracene or the phenanthrene, the compact all
6-ring PAHs, but that of the tolane molecule. The
strong S0-S2 phenantrene transition is clearly
absent. The spectrum of the C10H8 molecule is not
that of the bicyclic naphthalene, but probably
that of a monocycclic substituted benzene.
- -Identification of other species (C6D6 as
precursor ...) to gain deeper understanding of
the chemistry in plasmas - -Characterisation of other mixtures
- -Work in the visible.
Acknowledgment
References
1) F. Güthe H. Ding T. Pino J. P. Maier,
Chemical Physics, accepted. 2) T. Pino H. Ding
F. Güthe J. P. Maier, Journal of Chemical
Physics, 2208, 114, (2001). 3) http//www.chemie.u
nibas.ch/linnartz/ 4) W. Jenneskens, M. Sarobe,
Polycyclic Aromatic Compounds,. 14/15, 169
(1999). 5) C. Lifshitz, G. Reuben, J. Chem.
Phys. 50, 951 (1969).
The authors would like to thanks Georg Holderied
and Dieter Wild and the mechanical workshop for
their technical assistance. Tomasz Motylewski
and Danielle Furio (LPPM, Orsay France) are also
kindly thanked for their help in developing the
software of the experiment.