Lecture outline - PowerPoint PPT Presentation
1 / 40
Title:
Lecture outline
Description:
some of the things made possible/impacted by materials science. ... nucleus = housefly on center. Engineering Innovation |Materials. business. II. some chemistry ... – PowerPoint PPT presentation
Number of Views:97
Avg rating:3.0/5.0
Title: Lecture outline
1
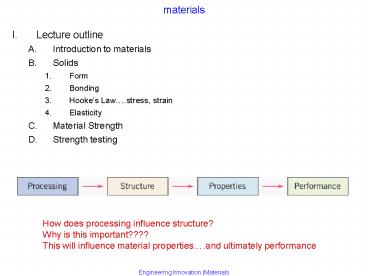
materials
- Lecture outline
- Introduction to materials
- Solids
- Form
- Bonding
- Hookes Law.stress, strain
- Elasticity
- Material Strength
- Strength testing
How does processing influence structure? Why is
this important???? This will influence material
properties.and ultimately performance
2
materials
some of the things made possible/impacted by
materials science.
3
what is structure? what is the basis of
structure?? a little chemistry is required at
this point.
4
business
- II. some chemistry
- A. protons, neutrons electrons ? atom
- what are atoms?
- smallest subunit of an element
- 2. protons neutrons ? nucleus
- 3. electron cloud
- of protons determines identity
- electrons protons (neutral)
- Electrons arranged in shells
- Electrons are the basis of materials properties
__________________________
________
_____
_______
atom stadium nucleus housefly on center
5
business
- II. some chemistry
- atoms
- 8. All atoms of a given element are identical
- 9. Atoms of different elements have different
masses - 10. a compound is a specific combination of atoms
of gt1 element - 11. in a chemical reaction, atoms are neither
created nor destroyed only change partners to
produce new substances
________
______
6
business
- II. some chemistry
- atoms
- 12. Can we see them?
- Yes
- electron microscopy or scanning probe microscopy
Xe on Ni
Xe on Ni
Au surface
Au surface
http//www.almaden.ibm.com/vis/stm/gallery.html
7
business
- II. some chemistry
- atoms
- 13. What can they do?
- a. form bonds with other similar atoms
elemental substances (molecules, metals, network
solids) - b. form bonds with atoms of other elements to
make compounds
sciences quest for simplicity.. various
combinations of the 100 elements make up all
matter on earth
http//www.almaden.ibm.com/vis/stm/gallery.html
8
materials
- III. what holds the atoms in a crystal/ceramic/pol
myer/elastomer together?........primary bonds - Covalent bonding
- Two or more atoms share electrons
- Strong and rigid
- Found in organics and sometimes ceramics
- Strongly directional
- E.g.methane CH4
- C has 4 valence electrons H has 1
- Elemental solids e.g. diamond
- Can be strong (diamond)
- Can be weak (Bi)
___________________
________________
9
materials
- III. what holds the atoms in a crystal/ceramic/pol
myer/elastomer together?........ primary bonds - ionic bonding
- Metal and non-metal
- Metal gives up valence electron(s) to non-metal
- Result is all atoms have a stable
configurationalso an electrical charge - E.g. NaCl-
- metal becomes ly charged (cation) non-metal
becomes ly charges (anion) - Electrostatic attraction
- Omnidirectional
- Close-packed
___________________
10
materials
- III. what holds the atoms in a crystal/ceramic/pol
myer/elastomer together?........primary bonds - C. metallic bonding
- Hold metals and alloys together
- Enables dense packing of atoms reason why
metals are heavy - Valence electrons (1, 2 or at most 3) not bound
to a particular atom - Free to drift throughout the entire material
sea of electrons - Nonvalence electrons atomic nuclei ion core
(net charge) - Good conductors of electrons heat
___________________
11
materials
- III. what holds molecules together?........seconda
ry bonds - 2ndry bonds are physical bonds and are weaker
than what weve just talked about - A. Hydrogen bonds
- Intermolecular attraction in which a H atom
bonded to a small, electronegative atom (N, O or
F)is attracted to lone pair of electrons on
another N, O or F - Weak
- Due to charge distribution on molecule
- Often seen in organic compounds
___________________
___________________
12
materials
- III. what holds molecules together?........seconda
ry bonds - B. Van der Waals forces
- Again, interactions are much weaker (10kJ/mol)
as compared to chemical bonds (100kJ/mol) - Forces arising from surface differences across
molecules - Gecko feet microscopic branched elastic hairs
on toes which take advantage of these
atomic-scale attractive forces to grip and
support heavy loads
___________________
Autumn et al. PNAS 2002, 99, 12252
13
materials
Takes a fictional superhero to bring nanotech to
the under 5s
14
Higher potential energy
Energy difference
Lower potential energy
15
materials
- IV. structure
- What do I mean by structure?
- Structure is related to the arrangement of a
materials components - This could be on any length scale
- Atomic, nano-, micro-, macro-
- All of these length scales matter
- Types of carbon (literally just carbon)
Carbon nanotubes
Diamond
Graphite
C60 - Fullerene
16
materials
- V. properties
- A material trait in terms of the kind and
magnitude of response to an imposed stimulus - e.g. sample subjected to force will experience
deformation - A polished metal surface will reflect light
- Categories of properties
- Mechanical, electrical, thermal, magnetic,
optical deteriorative - Each has a characteristic stimulus provoking a
response - mechanical properties relate deformation to an
applied load or force - mechanical properties include elastic modulus,
strength - Electrical properties (conductivity) respond to
an electric field - what causes differences in properties of
materials???
______
17
materials
- Many properties of a material are consequence of
- Identity of atoms that comprise them
- Spatial arrangement of those atoms
- Interactions between atoms
- atomic structure and bonding are important
18
materials
- Material properties
19
materials
Same material aluminum oxide. Depending on
structure (which is influenced by processing)
materials are transparent, translucent, opaque
20
materials
- VI. Solid materials
- Classification
- Crystals
- molecules attracted to one another try to cohere
in a systematic way, minimizing volume (dense
materials) - stiff yet ductile (capable of large amounts of
deformation without fracture) - Glasses/ceramics
- materials whose high viscosity at liquid/solid
point prevents crystallization amorphous - E.g. porcelain, SiO2, glass, cement
- Stiff, strong, hard BUT very brittle and
susceptible to fracture - insulators
- Polymers
- materials built up of long chains of simple
molecular structures plastics and living things - Low densities
- Extremely ductile, pliable can be formed into
complex shapes - Soften/decompose at high T
- Elastomers
- long-chain polymers which fold or coil e.g.
artificial rubber - Totally elastic due to cross-linking
21
materials
- solid materials
- Elastomers
Elastic deformation Partial uncoiling,
straightening elongation
Unstressed Amorphous Twisted, kinked, coiled
Removal of stress..spring back
silly putty smash
silly putty pull
22
materials
- VII. mechanical properties
- Lets think about spaghetti
- How easy is to break it by pulling (tension)?
- Is thicker spaghetti easier or harder to break by
pulling? - Theory says that force needed increases with
cross sectional area - How easily will it buckle if you compress the
ends? - Depends on force, material strength, length and
thickness of spag - A longer piece buckles easier than a shorter
piece - Thinner piece buckles easier than a thicker piece
- How easily will it bend if you push
perpendicular? - Is it tension, compression?
- Deflection depends on force, material strength,
length of span, area of spaghetti - Larger force, larger deflection
- For a given force, longer pieces bend easier
- For a given force, thin pieces bend easier
spaghetti crop
23
materials
- VII. mechanical properties
- How do engineers figure in the picture?
- 2 concepts stress and strain
- structural engineers determine stress/strain
distributions in objects subjected to
well-defined loads (beams in bridges) - materials/metallurgical engineers produce
materials that will have the desired mechanical
properties
___________________
24
materials
- VII. mechanical properties
- first need to define stress and strain
- 1. stress is related to the force or load
applied to a material - a. stress ? force/original area
- b. from figure ? F/A0 (units?)
- F newton kg m / s2
- ? F/A0 N/m2
- pascal N/m2
- MPa 106 Pa, GPa 109 Pa
- from figure ? F/A0 Pa or F/A0 x 10-6
MPa
____?____
__?__
25
materials
- VII. mechanical properties
- first need to define stress and strain
- strain is related to the response of the material
to the applied force - a. strain e change in length over original
length ?l/l0 - b. strain is unitless but m/m (or in/in) may be
used - strain can be expressed as a
- c. 2 types elastic plastic
strain/deformation, - (i) elastic strain exists only while stress is
applied - elasticity
- (ii) plastic strain does not disappear upon
removal of - stress plasticity
____?____
___?___
26
materials
- VII. mechanical properties
- C. compression stress/strain test
- 1. force is now in the opposite direction
- compressive force taken to be negative ? negative
stress - since l0 gt li, ? negative strain
- tensile tests are easier to perform
- very little additional information from
compressive tests - compressive tests more useful if
- (i) materials behavior under large and permanent
(plastic) strain is needed - (ii) material is brittle
____?____
__?__
27
materials
- mechanical properties
- End up with a stress-strain curve
- 1. provides huge amount of information about
material properties
28
materials
- mechanical properties
- End up with a stress-strain curve
- 2. Initial part of curve is especially
interesting..
Yield strength
Yield strength Load required to go
from elastic-plastic deformation
29
materials
- mechanical properties
- E. Hookes Law and Youngs modulus, E
- 1. stress (?) and strain (e) are proportional
under certain conditions (low stress) - a. ? eelE Hookes Law
- b. E - Youngs modulus, modulus of elasticity,
stiffness, resistance to elastic deformation (GPa
or psi) - c. physical meaning of E being large?
- higher E implies greater stiffness
____?____
_?__
Material range of E Metal 45 400
GPa Ceramics 60 500 GPa Polymers 0.01 4
GPa Spaghetti 4.8 GPa
_____?_____
30
materials
- mechanical properties
- microscopic description of elastic deformation
- 1. strain manifests as small changes in
interatomic spacing of bonds - 2. E is a measure of resistance to separation
of adjacent atoms/ions/ molecules (i.e. it is
related to bonding forces)
_______?_______
Or differences in E are due to differences in
bonding! In other works microscopic (bonding)
determines macroscopic (E) Also as T increases,
E generally decreases
___?___
31
materials
- mechanical properties
- G. Youngs modulus, E for different materials
- 1. Values of E for ceramics are similar to
metals for polymers E is lower - Why?
- 5. As temperature increases, E diminishes
_?__
32
2. mechanical properties of materials
materials
- mechanical properties
- H. tensile strength (TS)
- maximum load / initial area
- a. TS is the stress value at the maximum of
the s-s curve, point M - b. corresponds to maximum stress sustainable
by a structure in tension - c. if this stress is maintained, fracture will
result - d. All deformation so far is uniform
throughout speciman - 2. at point M, neck formation occurs
- 3. stress is concentrated at M
- 4. fracture ultimately occurs at F
__?__
__?__
youtube.com
33
2. mechanical properties of materials
materials
- mechanical properties
- I. ductility and elongation
- ductility is the degree of plastic deformation at
(prior to) failure - low or no ductility brittle
- ductility is quantified as elongation, EL
- (i)
- lf length at fracture
- l0 initial length
__?__
youtube.com
34
materials
- VIII. Material strength
- Tensile strength
- How hard does something need to be pulled to
break material bonds - Some examples
- Steel piano wire 450,000 psi
- Aluminum 10,000 psi
- Concrete 600 psi
- Compression strength
- Materials fail in compression in many ways
depending on geometry, support - Buckling hollow cylinders e.g. tin can
- Bending long rod or panel
- Shattering heavily loaded glass
- Yield strength
- Load required to cross line from elastic to
plastic deformation - Ultimate tensile strength
- Maximum possible load without failure
35
materials
- IX. Material testing
- A. Tensile strength most common method
- 1. apply stress uniaxially along sample
- continually increase force on ends
- perform test until fracture (sample breaks)
- measure force vs. sample elongation
- tensile testing machine elongates specimen at a
constant rate - applied load and resulting elongations are
continuously and simultaneously measured
steel 1018 stress-strain
36
2. mechanical properties of materials
materials
- IX. Material testing
- Aside..
- 1. in stress-strain plots it appears that
stress is decreasing between - M and F
- 2. it is not decreasing.any ideas what is
happening? - 3. cross-sectional area is decreasing in the
necking region - 4. results in a reduction in the load-bearing
capacity of specimen
youtube.com
37
materials
- IX. Material testing
- B. Euler buckling load, Pc
- P load (MLT-2)
- I moment of inertia (L4)
- E Youngs modulus (ML-1T2)
- L length (L)
- 4 variables, 3 primitive dimensions 1
dimensionless group
38
materials
- Material testing
- What if the material is very brittle.can we do a
tensile test? - Tensile tests cant easily be done on
ceramics/brittle material because - Difficult to prepare and test samples with
required geometry - Difficult to grip brittle materials without
fracturing them - Ceramics fail very quickly (0.1 strain)
- Transverse bending test is more usually employed
39
materials
- Material testing
- C. bending
40
materials
- Material testing
- Bending
- At point of loading, top surface is in
compression and bottom surface is in tension - Stress is computed from specimen thickness, the
bending moment, and the moment of inertia of
cross-section
41
materials
- Material testing
minimizing moments of inertia to increase rates
of rotation
42
materials
- Material testing
- Compressive strength
- whats going to happen a beam (spaghetti) under
compression? - Will fail by crushing or buckling, depending on
material and L/d - Crushing atomic bonds begin to fail, inducing
increased local stresses, which causes more bonds
to fail - Buckling complicated as there are many modes