SPECTROSCOPY PowerPoint PPT Presentation
1 / 28
Title: SPECTROSCOPY
1
SPECTROSCOPY
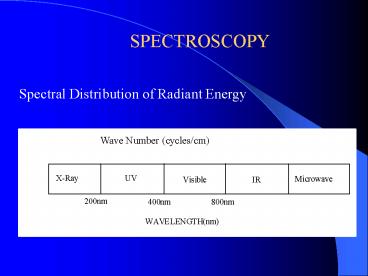
Spectral Distribution of Radiant Energy Wave
Number (cycles/cm)
2
SPECTROSCOPY
V Wave Number (cm-1) l Wave Length C
Velocity of Radiation (constant) 3 x 1010
cm/sec. u Frequency of Radiation
(cycles/sec) The energy of photon h
(Planck's constant) 6.62 x 10-27 (Erg?sec)
C u?
3
(No Transcript)
4
(No Transcript)
5
(No Transcript)
6
DISPERSION OF POLYCHROMATIC LIGHT WITH A PRISM
Prism - spray out the spectrum and choose the
certain wavelength (l) that you want by slit.
7
SPECTROSCOPY
1. Spectrophotometer - an instrument which can
measure the optical density of a sample at any
wavelength.
8
Fluorometer
2. Fluorometer - measures the intensity of
fluorescent light emitted by a sample exposed to
UV light under specific conditions.
9
BEER LAMBERT LAW
As the cell thickness increases, the intensity of
I (transmitted intensity of light ) decreases.
10
R- Transmittance R I0 - original
light intensity I- transmitted light
intensity Transmittance 100 x
Absorbance (A) or optical density (OD)
Log Log 2 - LogT
Log is proportional to C (concentration
of solution) and is also proportional to L
(length of light path through the solution).
11
A ? CL KCL by definition and it is called the
Beer Lambert Law. A KCL K Specific Extinction
Coefficient ---- 1 g of solute per liter of
solution A ECL E Molar Extinction
Coefficient ---- Extinction Coefficient of a
solution containing 1g molecule of solute per 1
liter of solution
12
E differs from K (Specific extinction
Coefficient) by a factor of molecular
weight. UNITS A ECL A No unit
(numerical number only)
13
L Cm C Moles/Liter A KCL A No unit
C Gram/Liter L Cm
14
STEPS IN DEVELOPING A SPECTROPHOTOMETRIC
ANALYTICAL METHOD
- Run the sample for spectrum
- 2. Obtain a monochromatic wavelength for the
maximum absorption wavelength. - 3. Calculate the concentration of your sample
using Beer Lambert Equation A KCL
15
SPECTROPHOTOMETR READINGS
16
ULTRAVIOLET SPECTRUM
17
There is some A vs. C where graph is
linear. NEVER extrapolate beyond point known
where becomes non-linear.
18
SPECTROMETRIC ANALYSIS USING STANDARD CURVE
Avoid very high or low absorbencies when drawing
a standard curve. The best results are obtained
with 0.1 lt A lt 1. Plot the Absorbance vs.
Concentration to get a straight line
19
CELLS
UV Spectrophotometer Quartz (crystalline
silica) Visible Spectrophotometer Glass IR
Spectrophotometer NaCl
20
LIGHT SOURCES
UV Spectrophotometer 1. Hydrogen Gas
Lamp 2. Mercury Lamp Visible Spectrophotometer 1
. Tungsten Lamp IR Spectrophotometer 1. Carborund
um (SIC)
21
CHEMICAL STRUCTURE UV ABSORPTION
Chromophoric Group ---- The groupings of the
molecules which contain the electronic system
which is giving rise to absorption in the
ultra-violet region.
22
CHROMOPHORIC STRUCTURE
Group Structure nm Carbonyl gt C
O 280 Azo -N N- 262 Nitro -NO 270
Thioketone -C S 330 Nitrite -NO2 230 C
onjugated Diene -CC-CC- 233 Conjugated
Triene -CC-CC-CC- 268 Conjugated
Tetraene -CC-CC-CC-CC- 315 Benzene 261
23
UV SPECTROMETER APPLICATION
Protein Amino Acids (aromatic) Pantothenic
Acid Glucose Determination Enzyme Activity
(Hexokinase)
24
FLUOROMETER APPLICATION
Thiamin (365 nm, 435 nm) Riboflavin Vitamin
A Vitamin C
25
VISIBLE SPECTROPHOTOMETER APPLICATION
Niacin Pyridoxine Vitamin B12 Metal
Determination (Fe) Fat-quality Determination
(TBA) Enzyme Activity (glucose oxidase)
26
EXAMPLES
1. A solution of purified DNA isolated from
Escherichia coli gives an absorbance of 0.793 at
260 Mm in a 1 Cm cell at pH 4.5. If E11Cm is
197, calculate the concentration of the solution
in milligrams per milliliter. 2. Calculate the
Molar Extinction Coefficient E at 351 nm for
aquocobalamin in 0.1 M phosphate buffer. pH
7.0 from the following data which were obtained
in 1 Cm cell. Solution C x 105
M Io I A 2.23 93.1
27.4 B 1.90 94.2 32.8
27
3. The molar extinction coefficient (E) of
compound x is 3 x 103 Liter/Cm x Mole If
the absorbance reading (A) at 350 nm is 0.9 using
a cell of 1 Cm, what is the concentration of
compound x in sample? 4. The concentration of
compound Y was 2 x 10-4 moles/liter and the
absorption of the solution at 300 nm using 1 Cm
quartz cell was 0.4. What is the molar
extinction coefficient of compound
Y? 5. Calculate the molar extinction
coefficient E at 351 nm for aquocobalamin in 0.1
M phosphate buffer. pH 7.0 from the following
data which were obtained in 1 Cm
cell. Solution C x 105 M I0 I A
2.0 100 30
28
Question 6.A 0.01E 10000L / mole x cmL
1cmA ECL0.01 10000L/mole X Cm X C
(Concentration) x 1CmC mole / LiterC X
mole / Liter X mole (236 g/mole) / Liter (1000
Cm3) x PPM (10-6 g/Cm3) X mole (236 g / mole) /
Liter x 1 Liter / 1000 Cm3 x ( PPM) 10-6 g /
Cm3)x PPMPPM 1ug / Cm3 1ug 10-6 g