What PowerPoint PPT Presentation
Title: What
1
(No Transcript)
2
(No Transcript)
3
Whats a phenolic compound? A secondary product
that contains a phenol group - a hydroxyl
functional group on an aromatic ring.
Phenolics are a chemically diverse group many
different properties and functions.
4
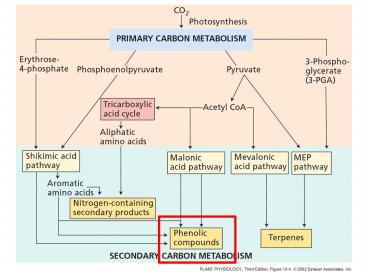
Biosynthesis of phenolics Shikimic acid pathway
is most common in plants. Converts simple
carbohydrates into aromatic amino acids. Not
present in animals.
5
Most plant phenolics are derived from cinnamic
acid formed from phenylalanine by phenylalanine
ammonia lyase (PAL) enzyme.
PAL activity is inducible fungal infection, low
nutrient levels, high light
PAL
6
Major types of phenolics 1. Simple phenolics -
e.g. coumarins 2. Lignin - 2nd most abundant
compound in plants 3. Flavonoids - two aromatic
rings, 2 pathways anthocyanins,
flavones/flavonols 4. Condensed tannins
polymerized flavonoids 5. Hydrolyzable
tannins made of phenolic acids and
sugars smaller molecules than condensed
tannins
7
1. simple phenolics
Fig. 13.10
Involved in defense against insect
herbivores and fungi, some may have
allelopathic function.
8
Simple phenolics
Caffeic acid ferulic acid implicated in
allelopathy. Psoralen is one of several
phototoxic furanocoumarins, (UV activated)
Fig. 13.11
9
Furoanocoumarins can certain light wavelengths.
(common in Umbelliferae family celery, parsnip)
Leaf rolling insects
- Insects usually roll leaves for protection from
predation and to create a habitat - Sometimes insects roll leaves to prevent
furanocourmarins from being activated
10
Furanocoumarins can bind to DNA or react with
lipids and proteins
11
Simple phenolics
Fig. 13.11
12
2. Lignin Second most abundant compound in
plants. Highly branched polymer of
phenylpropanoid groups (benzene-C3)
13
- often found in vessel elements, tracheids, and
stems confers structural support. Primary
structural - role!
- - Secondary role as a herbivore deterrent by
reducing digestibility of plant matter - also difficult for microbes to degrade its
presence slows litter decomposition.
14
3. Flavonoids - basic structure is two
aromatic rings joined by a 3C bridge.
- anthocyanins
- flavones
- flavonols
- isoflavonoids
Fig. 13.10
15
(No Transcript)
16
Fig. 13.13
Flavonoids
a) Anthocyanidins and anthocyanins are
pigments that give plant tissues red, blue, and
purple color. Pollinator attraction Disperser
attraction
17
(No Transcript)
18
Flavonoids continued
b c) flavones and flavonols UV
absorbing protection against UV (280 - 320
nm) insect pollinator attraction
How we see the golden eyes
How honeybees see golden eyes UV absorbing
flavonols are present in the inner part of petals
19
d) Isoflavonoids common in legumes antimicrobial
properties also involved in signalling e.g.
attracting rhizobia
Rhizobium is attracted to legumes through
signaling by isoflavanoids released from roots.
20
Tannins Condensed -formed by
polymerization of flavonoid units -common in
woody plants Hydrolyzable - contain phenolic
acids gallic acid, simple sugars - smaller
molecules than condensed tannins - more easily
hydrolyzed and degraded Tannins reduce growth
and survival of many different kinds of
herbivores Also act as antioxidants - eat your
isoflavonoids Johnny!
21
Many foods contains tannins (e.g. tea, red wine)
and have some healthy side effects for humans
(e.g. disallowing constriction of blood
vessels) Tannins also make protein less
digestible. Animals can sense high levels of
tannins in their food and opt for another food
resource (e.g. mule deer, beavers). High levels
of tannins in diet can actually kill some
animals.
22
Condensed tannins are polymerized flavonoids.
23
Hydrolyzable tannins are made of phenolics and
sugars.
Fig. 13.15
24
- The term tannin is derived from the tanning
process in which raw animal hides are preserved
by rubbing tannins on them. The tannins help to
complex the proteins and keep them from
degrading. - This protein-binding property of tannins lends
them their toxicity to herbivores. - tannins can bind digestion enzymes in the gut of
herbivores. - tannins also form complex polymers when bound to
proteins which are difficult to digest, thus
decreasing the nutritional value of the plant
material.
25
Tannins can reduce nutritional value of
tissuesby binding to proteins, making them less
digestible.
Fig. 13.16
Care for a spot of milk in your tea?
26
(No Transcript)
27
Creosote bush, Larrea tridentata Leaves are
10-25 phenolic resin. 40 of resin is NDGA
(nordihydroguaiaretic acid), remainder is
o-methylated flavones and flavonols. Deters
insect herbivory. Mammalian herbivores select
older leaves (less resin).
28
Active compounds in creosote leaves. NDGA and
similar compounds. Amino acids. Flavonoids.
Volatile oils. Triterpenes. Saponins.
USDA formerly used NDGA as an antioxidant to
prevent rancidity in food. Now known to cause
liver and kidney disease in lab
animals. Creosote has long been used in
traditional Native American and Mexican herbal
medicine
29
Creosote applications in herbal therapy To
dissolve urinary kidney stones. Anti
inflammatory for respiratory ailments (asthma)
and arthritis To eliminate gallstones
Against urinary infections For the treatment
of venereal disease As an abortifacient
Against diabetes Bronchitis and colds
Rheumatism Against some types of cancer As a
mouthwash against tooth decay and halitosis
30
- After life effects of phenolic compounds.
- Plant litter decomposition, and release of
nutrients - from decomposing litter, are strongly influenced
by - the chemical composition of the litter.
- Litter higher in tannins and lignin decomposes
more slowly.
Decomposition rate
Lignin/Nitrogen ratio
31
Bill Shakespeare, secondary chemist