Ethers - PowerPoint PPT Presentation
Title:
Ethers
Description:
Compounds that contain the oxygen atom in a ring are classified as cyclic ethers ... Skunk scent is caused by the two thiols shown below center and right. ... – PowerPoint PPT presentation
Number of Views:91
Avg rating:3.0/5.0
Title: Ethers
1
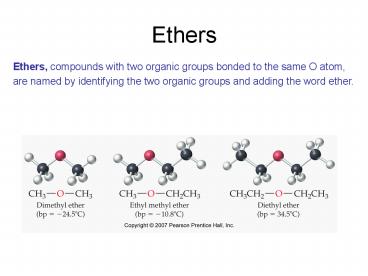
Ethers
Ethers, compounds with two organic groups bonded
to the same O atom, are named by identifying the
two organic groups and adding the word ether.
2
Compounds that contain the oxygen atom in a ring
are classified as cyclic ethers and are often
given common names.
3
Alkoxy groups
- An OR group is referred to as an alkoxy group
- -OCH3 is a methoxy group, -OCH2CH3 is an ethoxy
group, and so on. - These names are used when the ether functional
group is present in a compound that also has
other functional groups.
4
Other functionalities that are NOT Ethers
5
Thiols
Thiols, or mercaptans, are sulfur analogs of
alcohols. Skunk scent is caused by the two
thiols shown below center and right. The
systematic name of a thiol is formed by adding
-thiol to the parent name.
6
Thiols
- Thiols (R-SH) react with mild oxidizing agents to
yield a disulfide (R-S-S-R). - The reverse reaction (R-S-S-R ? 2R-SH) occurs
when a disulfide is treated with a reducing
agent. - Hair protein is rich in S-S and SH groups. When
hair is permed, some disulfide bonds are broken
and new ones are formed, giving hair a different
shape.
7
Halogen containing compounds
Any organic compound that contains a halogen atom
can be considered a halide. Alkyl halides are
organic compounds of the type R-X, containing an
alkyl group R covalently bonded to a halogen X.
Chlorofluorocarbons (CFC) are compounds
containing chlorine, fluorine and carbon only,
i.e they contain no hydrogen.
8
Halogenated organic compounds have a variety of
medical and industrial uses -Anesthetics -Sol
vents, propellants, degreasing agents -Fire
extinguishers -Herbicides, fungicides,
insecticides Despite the enormous benefits of
halogenated organic compounds, their use has been
restricted, and sometimes banned altogether
because -They persist in the environment and
are not broken down rapidly. -They
accumulate in some animals to harmful
levels. -They can damage the ozone layer.
Adapted from Pearson Prentice Hall Inc. 2007
9
Chlorofluorocarbons (CFCs)
CFCs-Prohibited/Restricted use because of their
role in Ozone Depletion Hydrochlorofluorocarbons
(HCFCs) are of a class of haloalkanes where
notall hydrogen has been replaced by chlorine or
fluorine. They are used primarily as
chlorofluorocarbon (CFC) substitutes, as the
ozone depleting effects are only about 10 of
the CFCs. Hydrofluoro compounds (HFCs) They
contain no chlorine. Lower global warming
potential than the HCFCs because of no known
effects on the Ozone layer. They do have activity
on other realms of greenhouse gases, which also
contribute to global warming.
10
Thyroxine
Tyrosine (amino acid)
Triiodothyronine (T3)
(T4)
11
Production of Thyroid Hormones
- T3 has three iodine atoms and T4 (Thyroxine)
contains 4 iodine atoms - Via a thyroperoxidase enzyme, Iodine molecules
are covalently bound - To Tyrosine residues
- These Tyrosine residues are known as
DiIodoThyronine and - MonoIodoThyronine
- Linking of these DIT and MIT residues forms T3
(Triiodothyronine) - And T4 Thyroxine