Neurotransmitters - PowerPoint PPT Presentation
1 / 16
Title:
Neurotransmitters
Description:
1. Neurotransmitters. Properties of neurotransmitters: 1) synthesized in the presynaptic neuron ... 5) Presence of receptor on the post-synaptic neuron. ... – PowerPoint PPT presentation
Number of Views:130
Avg rating:3.0/5.0
Title: Neurotransmitters
1
Neurotransmitters
R.E.B, 4MedStudents.com, 2003
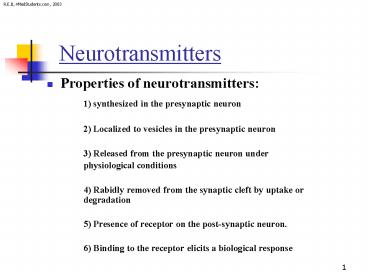
- Properties of neurotransmitters
- 1) synthesized in the presynaptic neuron
- 2) Localized to vesicles in the presynaptic
neuron - 3) Released from the presynaptic neuron under
- physiological conditions
- 4) Rabidly removed from the synaptic cleft by
uptake or degradation - 5) Presence of receptor on the post-synaptic
neuron. - 6) Binding to the receptor elicits a biological
response
2
(No Transcript)
3
Neurotransmitters found in the nervous system
EXCITATORY Acetylcholine Aspartate Dopamine Hi
stamine Norepinephrine Epinephrine Glutamate S
erotonin INHIBITORY GABA Glycine
4
Acetylcholine synthesis
- In the cholinergic neurons acetylcholine is
synthesized from choline. This reaction is
activated by cholineacetyltransferase
As soon as acetylcholine is synthesized, it is
stored within synaptic vesicles.
5
Release of acetylcholine from presynaptic neurons
- 1)When the nerve impulse (Action potential) moves
down the presynaptic axon to the terminal bulb
the change in the membrane action potential
causes the opening of voltage gated calcium
channels open allowing Ca2 ions to pass from the
synaptic cleft into the axon bulb. - 2) Within the bulb the increase
- in Ca2 concentration causes the
- synaptic vesicles that contain
- acetylcholine to fuse with the
- axonal membrane and open
- spilling their contents into
- the synaptic cleft.
6
Binding of acetylcholine to the postsynaptic
receptors
- The postsynaptic membrane of the receptor
dendrite has specific cholinergic receptors
toward which the neurotransmitter diffuses.
Binding of acetylcholine trigger the opening of
ion channels in the postsynaptic membrane
initiating action potential that can pass in the
next axon.
- Acetylcholine receptors
- Acetylcholine receptors are ion channels
receptors made of many subunits arranged in the
form (a2)(ß)(?)(d). - When Acetylcholine is not bounded to the
receptors, the bulky hydrophobic leu side close
the central channels preventing the diffusion of
any ions. - Binding of two acetylcholine molecules to the
receptors will rotate the subunits in which the
smaller polar residues will line the ion channel
causing the influx of Na into the cell and
efflux of K resulting in a depolarization of the
postsynaptic neuron and the initiation of new
action potential.
7
Removal of Acetylcholine from the synaptic cleft
- In order to ready the synapse for another
impulses - 1) The neurotransmitters, which are released
from the synaptic vesicles, are hydrolyzed by
enzyme present in the synaptic cleft
Acetylcholinestrase giving choline, which
poorly binds to acetylcholine receptors. - Acetylcholine H2O Choline H acetate
- 2) The empty synaptic vesicles, which are
returned to the axonal terminal bulb by
endocytosis, must be filled with acetylecholine.
8
Structure of AchE
- Acetylcholinesterase (AchE) is an enzyme, which
hydrolyses the neurotransmitter acetylcholine.
The active site of AChE is made up of two
subsites, both of which are critical to the
breakdown of ACh. The anionic site serves to bind
a molecule of ACh to the enzyme. Once the ACh is
bound, the hydrolytic reaction occurs at a second
region of the active site called the esteratic
subsite. Here, the ester bond of ACh is broken,
releasing acetate and choline. Choline is then
immediately taken up again by the high affinity
choline uptake system on the presynaptic
membrane.
9
Catecholamine Synthesis (Dopamine, Norepinephrine
and Epinephrine).
- 1) First Step Hydroxylation
- In this step the reaction involves the
conversion of tyrosine, oxygen and
tetrahydrobiopterin to dopa dihydrobiopterin.
This reaction is catalyzed by the enzyme tyrosine
hydroxylase. It is irreversible reaction. - 2) Second step Decarboxylation
- In this step the dopa decaboxylase will catalyze
the decaoxylation of dopa to produce dopamine.
The deficiency of this enzyme can cause
Parkinsons disease. It is irreversible
reaction. The cofactor in this reaction is the
PLP (pyridoxal phosphate). In the nerve cells
that secrete dopamine as neurotransmitter the
pathway ends at this step.
10
Catecholamine Synthesis (Dopamine, Norepinephrine
and Epinephrine).
3) Third step Hydroxylation This reaction is
catalyzed by the enzyme dopamine ß- hydroxylase.
The reactants include dopamine, O2 and ascorbate
(vitamin C). The products are norepinephrine,
water and dehydroascorbate. It is an irreversible
reaction). The end product in noradrenergic
cells is norepinephrine and the pathway ends
her. 4) Forth step Methylation This reaction
is catalyzed by phenylethanolamine
N-methyltransferase. Norepinephrine and
S-adenosylmethionin (ado-Met) form epinephrine
and S-adenosyl homocysteine (ado-Hcy).
11
(No Transcript)
12
Serotonin synthesis
- Serotonin is synthesized from the amino acid
Tryptophan. - The synthesis of serotonin involve two reactions
- 1) 1) Hydroxylation
- Tryptophan 5- Hydroxytryptophan
- The enzyme catalyzes this reaction is Tryptophan
Hydroxylase. - The Co- factor is Tetrahydrobiopterin, which
converted in this reaction to Dihydrobiopterin. - 2) 2) Decarboxylation
- 5- hydroxytryptophan Serotonin
- The enzyme is hydroxytryptophan decarboxylase.
- Serotonin is synthesized in CNS, Chromaffin
cells.
13
(No Transcript)
14
Break down of serotonin
- Serotonin is degraded in two recations
- 1) Oxidation
- 5-hydroxytryptoamine O2 H2O
5- Hydroxyinodole-3-acetaldehyde - 2) Dehydrogenation
- 5- Hydroxyinodole-3-acetaldehyde
5-hydroxindole-3-acetate -
(Anion of
5-hydroxyindoleacetic acid)
- Aldehyde dehydrogenase
15
Other Neurotransmitters
16
Summary