Important Criteria for HPLC Detectors - PowerPoint PPT Presentation
1 / 25
Title:
Important Criteria for HPLC Detectors
Description:
... Mobile Phase Composition --For example, if UV-detector is used in HPLC changing the M.P composition ... Careful choice of M.P. pH and M.P composition quench If ... – PowerPoint PPT presentation
Number of Views:437
Avg rating:3.0/5.0
Title: Important Criteria for HPLC Detectors
1
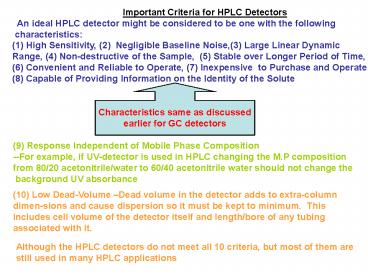
- Important Criteria for HPLC Detectors
- An ideal HPLC detector might be considered to
be one with the following - characteristics
- High Sensitivity, (2) Negligible Baseline
Noise,(3) Large Linear Dynamic - Range, (4) Non-destructive of the Sample, (5)
Stable over Longer Period of Time, - (6) Convenient and Reliable to Operate, (7)
Inexpensive to Purchase and Operate - (8) Capable of Providing Information on the
Identity of the Solute
Characteristics same as discussed earlier for GC
detectors
(9) Response Independent of Mobile Phase
Composition --For example, if UV-detector is used
in HPLC changing the M.P composition from 80/20
acetonitrile/water to 60/40 acetonitrile water
should not change the background UV absorbance
(10) Low Dead-Volume Dead volume in the detector
adds to extra-column dimen-sions and cause
dispersion so it must be kept to minimum.
This includes cell volume of the detector itself
and length/bore of any tubing associated with
it.
Although the HPLC detectors do not meet all 10
criteria, but most of them are still used in
many HPLC applications
2
Major Types of HPLC Detectors
3
UV-Vis Absorbance Detector
Most commonly employed detector in HPLC awa.
Workhorse Detector for HPLC
Principle of Operation Operates on exactly same
principle as UV-Vis Spectrophotometer
- Light from the lamp passes through a UV
transmitting flow cell (through which - M.P flows and is connected to the column) and
falls on a diode measures the light - intensity I.
b) Usually, light from the lamp is also directed
to the reference diode for measurement of light
intensity I0
c)The detector electronics than convert the
signal from the two diodes into absorbance A,
which is transmitted to the data system and is
measured A log I0/I (ratio of the
intensity of absorption b/w ref and sample diode)
d)Analyte concentration (C) in the flow cell is
related to the absorbance of the analyte (A),
molar absorptivity (e), and flow cell length
(Lfc) by Beers Law
A e Lfc
4
What are the two general criteria in selecting
sensitive conditions that can be use to maximize
signal of sample components of interest in UV
detection in HPLC?
- Selection of Suitable Wavelength (l)
- --l max is ideal to work but a careful knowledge
of UV-Spectra is necessary - --UV spectra should be measured if standards are
available - --Diode-Array detector provides spectra of eluted
peak
b) Select l where sample have minimum absorption
interferences from matrix/solvent ---Usually
wavelength gt240 nm is best
5
- The figure to the right shows a UV-spectra of
Azo- - benzene(Az, concentration 3.73 x 10-3g/10mL and
- phenanthrene (P, 3.23 x10-3g/10mL) both recorded
in iso- - octane on a standard UV/Vis spectrophotometer.
What - wavelength would you select on your HPLC
detector? - Assume that Az is the contaminant in
- the sample and you are only interested in
- sensitive detection of P, what l would
- you choose for detecting P without
- detecting Az? and Why?
251 nm
It cannot be done but at 251 nm the ratio of A
and e of P to Az would be greatest.
342 nm
(ii) Assume that you have purified your sample
and now it contains only P and you want to
determine the concentration of P carefully in
your sample. Which l would you choose for
quantitation of P?
For sensitive detection, 251 nm is ideal for P.
For quantitation we must choose a l, where A or e
changes with l is not rapid (choose plateau) 261
nm is better.
(iii) Assume that P is the contaminant in the
sample and you are only interested in sensitive
detection of Az, what l would you choose for
detecting Az and Why?
The shoulder at 342 nm
(iv) Assume you are interested in detecting both
Az and P after the HPLC separation. Which l
would you choose? And Why?
300 nm (270-320 nm) for precise quantitation as
Beers Law is followed.
6
Fixed Wavelength Detectors --is the most common
and inexpensive detector. The use of suitable l
is determined by the nature of the light source
used as shown in Table below
--The above source (Hg, Cd, Zn and Mg) mention in
table shows sharp emission lines at l indicated
in the above table. These ls can be used for
sample that absorbs strongly at these wavelengths
--Deuterium lamp can be used over a range of
wavelength (covers a continuum of wavelengths),
hence covering most of the UV spectral region
Variable Wavelength Detectors (provide detection
of eluted peak at any selected l. --Less
sensitive than fixed wavelength but the detection
wavelength can be varied
--Deterium source is mostly used because it
provides continuum source. This can be combined
with a suitable monochromator in dual beam mode.
7
Photodiode Array Detectors (PDA) or DAD
--Even much more rapid scanning of the absorption
spectra of the eluted peak is possible using a
photodiode array detector --The optical
arrangement of the photodiode array detection is
shown below
--Optical arrangement is referred to as reverse
optics. This is because the dispersion device
(holographic gratings) is placed after the flow
cell (opposite to UV-Vis)
- Working of DAD
- Light from a continuum source (e.g., D2
- Lamp) passes through a lens system which
- focusses polychromatic light onto the
- flow cell (containing the sample)
b) The transmitted light then falls on a
holographic gratings where it is dispersed into
a photodiode array (PDA).
c)PDA is a several hundreds of photodiodes
arranged in a linear fashion. A typical
photodiode array has 512 diodes to cover a
range of wavelength (190-800 nm), each
photodiode has a bandwidth of 2 nm.
d) A range of wavelengths of light falls on a
photodiode array and each diode picks up a
different wavelength of light.
8
Name one advantage and one disadvantage of DAD
over single l detector in HPLC? Advantage DAD
provides absorption spectra of each peak and can
be used for peak purity analysis
Disadvantage DAD is less sensitive and more
expensive than single l detector
Applications of DAD a) A 3-D spectra of each peak
eluting from the column can be obtained
9
(b) Peak Purity Analysis
- --A chromatogram is shown with 5 peaks and
spectra is taken for peak a and - b at three points
- half-way up the rising side (i.e., the leading
edge of the peak) - (ii) top of the peak
- (iii) Halfway down the trailing side (i.e
trailing edge of the peak
Which peak a or b is pure?
Peak a is impure and peak b is pure. This is
because in peak b the Absorption spectra at
each points of a peak matches the l and does not
occurs for peak a
10
ELECTROCHEMICAL DETECTION IN HPLC
--Electrochemical detection (ECD) is a range of
detection techniques, which involves the
application of electric field (via a suitable
electrode) to a sample solution, followed by
measurement of resultant current.
--ECD includes the technique of Voltammetry,
Amperometry and Coulometry.
--Common characteristics in ECD A chemical
reaction (e.g., Faradaic oxidation or reduction
occurs) during ECD. To be capable of ECD solutes
much be easily oxidize or reduced. One example
is
--Basic Instrumentation for ECD
Detector is assembled using four basic components
- Potential power supply
- --Used for application of voltage
(2) Appropriate circuitory (amplifier for
measurement of current).
(3) A suitable flow-through sample cell. The
cell consists of three electrodes
(a)Working electrode (WE) potential is applied.
WE is glassy carbon or a precious metal (Au, Ag,
Pt), which is located in a suitable flow cell
through which M.P flows
(b) Auxillary electrode which measures the
flowing current (c) Reference electrode in
contact with electrolyte and sample solution
11
(4) Current-Voltage convertor Convert signal
generated by oxidative or reductive current back
to voltage to be read by a recorder
- Voltammetry detection. If the applied voltage is
varied over the course of - measurement and we measure the current resulting
from retention (Oxid/Red) of - analyte species than the ECD is called
Voltammetry
2. Amperometry detection. If a fixed voltage is
applied over the course of measurement and we
measure the current resulting from reaction of
analyte species than the ECD is called
Amperometry.
--Surface area of the working electrode is quite
small (0.5 cm2 or less)
--With small surface area faradaic reaction of
analyte is incomplete---only a fraction of the
analyte reacts --------? lt10 of analyte reacts
in a flow cell
3. Coulometric detection. If a fixed voltage is
applied over the course of measurement and we
measure the current resulting from reaction of
analyte species than the ECD Is called
Coulometry.
So what is the difference between Amperometry and
Coulometry? --Coulometry employs working
electrode of large surface area (gt 0.5 cm2),
this result in a complete and quantitative
reaction of the analyte at the electrode surface.
Thus, amperometry and coulometry can be
differentiated on the basis of Faradaic reaction
at the working electrode
12
Current-Potential Curve for two Electroactive
Solutes
The Figure to the right show the
current- potential curve for ascorbic acid and an
organic disulfides.
--The potential is applied and the current
produced is measured. -?Oxidation and Reduction
of electroactive species results in different
directions of the current flow based on this we
can generate oxidation or reduction current.
Oxidation current (Anodic Current) When compound
is oxidized the oxidation current flows out of
the electrode giving oxidation (anodic)current
which is negative. For e.g., ascorbic acid is
readily oxidized giving an anodic current at
0.23 V due to oxidation of enediol system.
Reduction current (Cathodic Current) When
compound is reduced, reduction electrons flows
into the electode giving (cathodic) current or
cathodic peak, which is positive. For e.g.,
organic disulfides are reduced giving a cathodic
peak at -10 V due to reduction.
13
To use reduction as a method of ECD in HPLC is
more difficult than using oxidation as a method
of ECD, why?
--Because M.P present in HPLC may have dissolve
oxygen (O2)
--O2 if present in the M.P is very easily reduced
and create background current, which is much
larger than the current produced by reduction of
analytes.
--Traces of O2 has to be removed carefully if ECD
in reductive mode is possible.
Application of ECD ECD is useful for organic
molecule containing functional group capable of
being oxidized or reduced. Some typical
functional groups sensed by electrochemical
detectors are shown to the right
How can one tell if the electroactive species
(species that can be electrochemically oxidized
or reduced) has the potential to detected in ECD?
If the applied voltage gt Ehalf of
the electroactive species then ECD of that
species is possible
14
- Disadvantages of ECD
- Technique is not very suitable for
electroreducible compounds - This is because of high background current which
is generated by dissolve - O2 in the M.P.
- Therefore, both M.P and the sample needs to be
highly degassed before use
2) Metal ion impurities interfere for
electroreductible compound in ECD
15
(No Transcript)
16
What kind of molecules show fluorescence?
Molecules with delocalized p-electrons (e.g.,
benzoapyrene)
17
FLUORESCENCE DETECTION IN HPLC
Background --From our background in spectroscopy
we know that molecules have different
energy states. The two important states are
Ground State Excited State
What happened when light energy is absorbed by a
molecule? --Energy of a molecule increases and
the molecule is promoted to excited state. This
is called Absorption or Excitation.
What happens when energy is released by a
molecule? --Energy of molecule decreases and the
molecule returns to ground state from Excited
state. This is called Emission
Hence, there are two steps in measurement of
fluorescence Fluorescence molecule absorbs
radiation at one l and emit radiation at a longer
l.
18
Fundamental Equation for Fluorescence
Detection --Because fluorescence is an optical
technique, it is also subjected to Beers Law.
For dilute solutions where ecl lt0.01, the
fluorescence intensity (If) can be written as
If 2.303
ff I0 ecl
-A linear relationship exist between If and
concentration (c) of the solute provided that ecl
is small
--What does the above equation tell us? --If can
be increased (i.e., S/N can be improved) by
working at higher ff, and high excitation power
Q. Why lasers instead of lamp provide high
sensitivity of detection in HPLC?
--Lasers produce high excitation power
(I0) --Laser light is monochromatic (little loss
of incident light)
19
Schematic of a Fluorescence Detector
--To avoid problems of differentiating between
excitation and emission fluorescence detector
operates in right angle configuration
- Working
- Radiation from a xenon or
- deuterium lamp passess through
- An excitation filter, which provides
- essentially monochromatic light)
- of desired wavelength to excite the
- sample.
2. This excitation l of light then passes
through the column effluent in the flow cell.
- When the sample molecule passes
- through the column effluent they are excited
- and emit light (fluorescence) at a longer l.
4. A second (emission) filter is positioned at
900 to the first filter to collect the emitted
light. In this way only the light emitted from
the sample fluorescence will pass on PMT for
quantitation of the emission signal
20
Advantages of Fluorescence Detection in HPLC
- Inherent advantage is higher sensitivity
- 2-3 orders of magnitude greater than UV-
- Detection. For example, polycyclic aromatic
- hydrocarbons (PAHs) are important air pollutant
- (needs to be detected at low concentration).
- Chromatogram on the right
- Compares the UV and fluorescence
- detection.
2. Derivitization with fluorescent reagent
o-phthaldehyde will enhance the detectability
21
Disadvantage of Fluorescence Detection in HPLC
Careful choice of M.P. pH and M.P composition
quench If For e.g., aniline is cationic at
acidic pH and do not fluoresce, but in pH range
of 7-12 it exist as a neutral species and
fluoresce
Dissolve oxygen and impurities in M.P also
quench fluorescence resulting in self absorption
22
REFRACTIVE INDEX DETECTION IN HPLC
-Closest to the ideal of a universal detector
(show respond to most solutes)
--Magnitude and the direction of the response
depends on the difference in RI between M.P and
the solute(s) Therefore, sensitivity reaches a
maximum when DRI is greatest
Is RI is a bulk or solute property detector?
It is a bulk property detector
- Output of RI detection may show a positive or a
negative peak in the same run - for several compounds. If the alkanes in the
above table are analytes separated - by HPLC using tetrahydrofuran as the M.P. Which
alkane in RI detector will show - positive, negative or no peak.
Nonane No peak
Pentane negative
Decane positive
23
- Types of RI detector
- --Two major types are available
- Deflection type (most popular)
- Reflection type (measure changes in reflected
light at glass-liquid interface - Reflection type is less popular than deflection
type RI detectors
Deflection type RI detector. The reason its
called deflection type is because deflection is
created in a rectangular sample cell by
separating the compartment into two parts with
a diagonal glass divider.
Operating principles --Light from the source
is focussed onto the sample cell, which consist
of sample and the reference chamber.
b) After deflection from the mirror, light is
diverted through an optical zero adjustment
(beam splitter) into the detector, which actually
consist of two photo- cells, P1 and P2.
c) When a solute elutes off the column the RI of
the sample compartment changes
d) This causes a change in the amount of
deflected light, which in turn changes the
relative the relative amount falling on P1 and
P2. Therefore, differences in relative output
P1 and P2 is measured.
24
Key Beam splitter movement, which is
proportional to the difference in RI that cause
the splitter to change its angle. The difference
is amplified by the amplifier and the change is
measured by the recorder.
What is the most important factor, which
influence the performance of RI detector?
Temperature has a profound effect on RI detection
signal. A small change in temperature (e.g.,
0.001 0C) cause a change in 10-6 RI units.
--Most commerical detectors have heat sink and
temperature control facilities, but this may
lead to undesired dead volume. Hence, N is
affected.
--Other important advantages of RI detector is
Universal (respond to all types of compounds).
Therefore, nochromophoric analytes
(carbohydrates, alcohols and polymers) can be
detected using RI detection.
- Disadvantages of RI detector
- Not suitable for gradient elution.
- Changes in solvent composition
- change RI. Therefore, baseline shifts
- and S/N is affected.
b) Require careful control of the column and
detector temperature.
c) Moderate sensitivity 10-9--?10-10g. Not
useful for trace analysis
25
(No Transcript)